-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2015; 5(5): 97-102
doi:10.5923/j.plant.20150505.01
Senna singueana Seed Viability and Root Suckering Capacity in Morogoro, Tanzania
1Sokoine University of Agriculture (SUA), Chuo Kikuu, Morogoro, Tanzania
2Institute of Scientific and Technological Research (IRST), Butare, Rwanda
3African Forest Forum (AFF), Nairobi, Kenya
Correspondence to: Nduwayezu J. B., Sokoine University of Agriculture (SUA), Chuo Kikuu, Morogoro, Tanzania.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Two studies were carried out during the 1996-1998 period to evaluate the effect of the applied pre-treatments (i.e. soaking seeds in cold water and direct seed sowing without pre-soaking in water) on Senna singueana seed germination rate and to assess the suckering capacity of this valuable indigenous shrub (i.e. fertilizer source). Thirteen seed testing dates (after 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12 months of seed storage) were involved in this study, and at each date, the number of seeds that germinate daily (i.e. 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12 days) was recorded and used to estimate the germination rate. Four sets of 100 seedlings raised in 10 cm x 15 cm polythene tubes and root-pruned 3 times (at 4, 5 and 6 months after seed sowing) were involved in the study on suckering ability of Senna singueana. The number of root suckers and subsequent survivors (percentage of success) were used to estimate both the root suckering capacity (%) and survival rate (%). Senna singueana is a lesser-known indigenous shrub found to be successfully propagating by both seeds and root suckers from young seedlings. The seeds of this species have a maximum germination rate of 80% with a viability period of up to one year but after 3 months it drops rapidly to less than 2% at 12 months of storage. No seed pre-treatment is required for optimum germination. In order to ensure a high germination rate (> 70%) the seeds of Senna singueana should not be stored for periods in excess of 3 months. Senna singueana seedlings sucker readily (with the survival rate of new root suckers ranging between 78-84%.) and the suckering capacity increases with the plant (root) size (r = 0.7183). The optimum size which maximizes suckering has not, however, been established. Research is urgently required to determine this including the ascertainment of whether suckering potential is confined to the tape roots alone or spread evenly to the lateral roots.
Keywords: Senna singueana, Seed germination, Seedling, Pruning, Root suckers, Fertilizer source
Cite this paper: Nduwayezu J. B., Senna singueana Seed Viability and Root Suckering Capacity in Morogoro, Tanzania, International Journal of Plant Research, Vol. 5 No. 5, 2015, pp. 97-102. doi: 10.5923/j.plant.20150505.01.
Article Outline
1. Introduction
- Senna singueana is an indigenous shrub with high agroforestry potentials (i.e. fertilizer source) belonging to the Caesalpinioideae sub-family, Fabaceae family of the super family Leguminoseae [1, 2, 3].The success of tree planting in agroforestry systems with the intention of improving soil fertility in smallholder farms has largely been attributed to the quality of the planting stock [4]. Senna singueana and other agroforestry trees and shrubs can be propagated either sexually (using seeds) or asexually (using vegetative materials). While considerable data are available on seed viability and propagation techniques of different multipurpose trees and shrubs [5], the published information on how to conserve the germplasm of Senna singueana is generally lacking. Propagating forest trees and agroforestry species by seeds was reported to be associated with inefficiency of use mainly caused by physical, chemical, morphological/ physiological and mechanical dormancy problems (i.e. difficulties in seed germination) [6-10] and rapid loss of viability [5]. Pre-treating seeds of a number of tree/shrub species with the aim of breaking the seed dormancy using a variety of methods generally enhanced the germination rate [6, 11-17, 5, 18, 10], but the degree of enhancement or response to the pre-treatment methods depended upon the type of tree and shrub species involved probably due to the high genetic variations between different plant species. A wide range of studies have demonstrated some advantages associated with tree propagation using vegetative materials as compared with the sexual propagation method [19, 20, 21, 10] and the importance of vegetative propagation in a number of plant species [22, 7, 5, 10] but the potential of Senna singueana to propagate vegetatively either through root suckers or using other vegetative materials has not yet been studied. Various studies on vegetative propagation of different plants have clearly demonstrated the superiority of plant species propagation using root suckers over other sexual and asexual methods [23-27]. The success of root suckers, however, can strongly be influenced by the plant biological, environmental and climatic conditions [28, 29, 30, 5, 31]. Knowledge about Senna singueana flowering pattern, seed germination and seed viability longevity may help the resource poor farmers with the provision of the germplasm of this valuable indigenous species for improved crop production. The main objective of the present study was, therefore, to determine the seed viability longevity and root suckering capacity of Senna singueana (source of fertilizer for smallholder farmers) with the intention of improving soil fertility and crop productivity in smallholder farms.
2. Materials and Methods
2.1. Study Sites Description
- These two studies were carried out at Kitete Village (37°8'E and 6°30'S; 375 m a.s.l) which lies within the Mkata plain in Kilosa District and Sokoine University of Agriculture (latitude: 5°40'S; longitude: 37°39'E; altitude: 525 m above the sea level) located at 3.0 km from the centre of Morogoro Municipality, which is about 200 km west of Dar es Salaam in Morogoro, Tanzania.
2.2. Experimental Procedures
2.2.1. Senna singueana Seed Germination
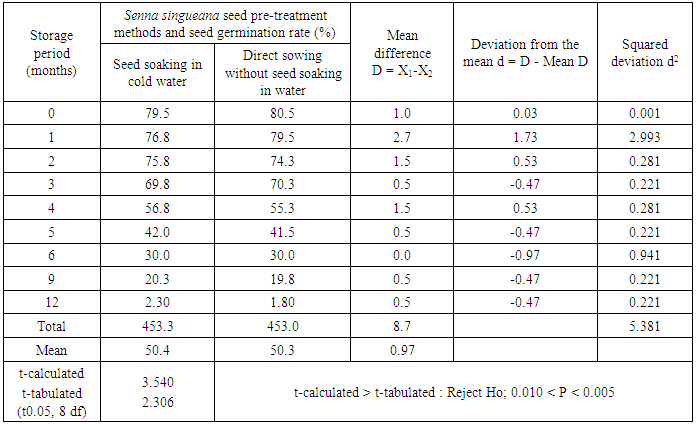
- The seeds of Senna singueana used in the plant propagation studies (propagation by root suckers and seeds) were collected from the local trees within the Kitete Village and from the Sokoine University of Agriculture Horticultural Unit during the December 1996 and November 1997 periods respectively. The seed storage and germination experiments were established in November 1997 in a completely randomized block design of 2 treatments (seed pre-treatment methods), 4 replications and 13 seed testing dates (after 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12 months of seed storage). The two treatments involved were soaking Senna singueana seeds in cold water and direct seed sowing without pre-soaking in water.Immediately after Senna singueana seed collection and processing, some seeds were tested for their germination rate (i.e. seed germination test at 0 month after seed collection). A duplicate of the remaining seeds was packed in two clean and dry cotton bags. One seed lot was stored for a period of one year (12 months) in a dry, clean and insect free place at Kitete Village and the other lot was stored for the same period under similar conditions in the Forest Biology Laboratory at Sokoine University of Agriculture. The subsequent seed germination tests out of the stored seed lots were carried out every after 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12 months of storage by taking 100 seeds of Senna singueana (25 seeds per replicate) and germinating them in sand. The number of seeds that germinate daily (i.e. 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12 days) was recorded. At the end of seed germination period (day-12), the germination rate (%) per each seed storage period for each seed lot was calculated as follows:
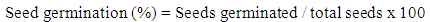 | (1) |
2.2.2. Senna singueana Vegetative Propagation by Root Suckers
- A 7-months study on Senna singueana seedling root suckering ability was carried out in both Kitete Village and Sokoine University of Agriculture nurseries during the December 1996- June 1997 period. The experiment was laid out in a completely randomized block design of 4 sets of healthy seedlings, 4 replications and 3 seedling pruning periods (4, 5 and 6 months after seed sowing). Four sets or observation units (U) of 100 seedlings each (i.e. seedlings raised in 10 cm x 15 cm polythene tubes) were initially arranged in the replication blocks of Compartment A (Figure 1). Polythene tubes containing 100 seedlings were randomly distributed in each block of each compartment. Compartments B and C were initially empty while waiting for root pruned seedlings from Compartment A.
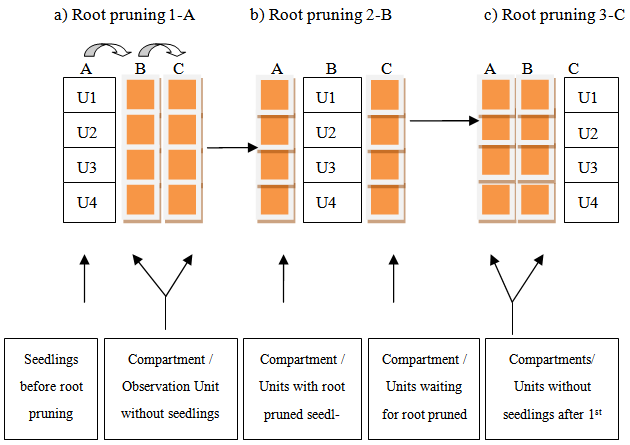 | Figure 1. Schematic representation of Senna singueana seedling transfers from one observation unit (U) of each Compartment to another unit after root pruning |
3. Results and Discussions
3.1. Senna singueana Seed Germination and Optimum Storage Period
- It is apparent from Figure 2 that, initially, the Senna singueana seed germination rate decreased gradually from an average maximum rate of 80% to 70% during the first 3 months of seed storage. After 3 months of storage however, a marked decline in germination rate (i.e. from 70% to less than 2% at 12 months of seed storage) was recorded. No statistical significant (P=0.05) differences in terms of germination rate were observed between the two Senna singueana seed pre-treatment methods (Table 1).
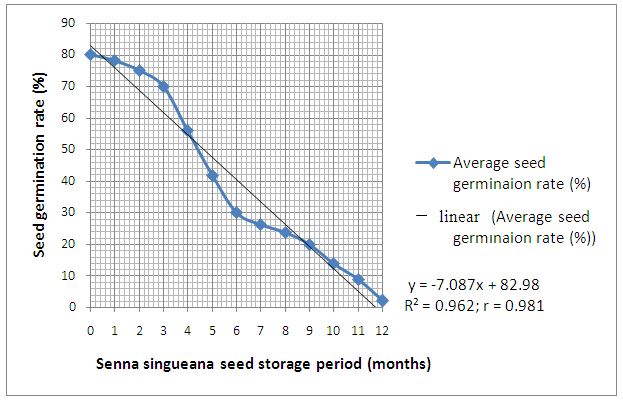 | Figure 2. Senna singueana seed germination rate at different storage periods (1997-1998) in Morogoro, Tanzania |
|
3.2. Senna singueana Growth and Root Suckering Capacity
- As shown in Figure 3, Senna singueana was successfully propagated through root suckers but the suckering ability of its roots increased with increasing size or growth period of the parent seedlings. It was also observed that the survival rate of the new seedlings or root suckers ranged between 78-84%.
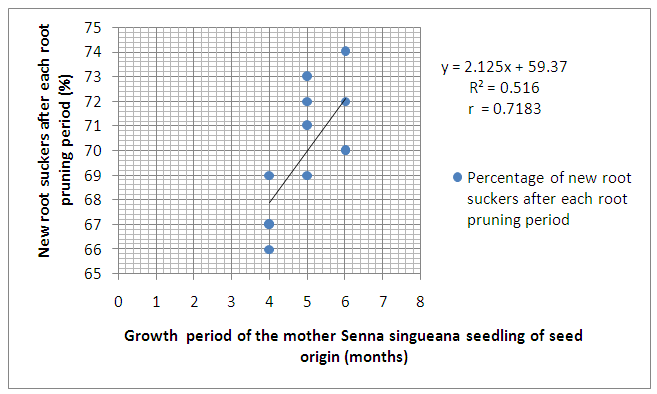 | Figure 3. Relationships between the overall mean of new Senna singueana root suckers and growth period of their mother seedlings of seed origin |
4. Conclusions and Recommendations
- • Senna singueana is a lesser-known indigenous shrub found to be successfully propagating by both seeds and root suckers from young seedlings.• The seeds of this species have a maximum germination rate of 80% with a viability period of up to one year but after 3 months it drops rapidly to less than 2% at 12 months of storage.• No seed pre-treatment is required for optimum germination.• In order to ensure a high germination rate (> 70%) the seeds of Senna singueana should not be stored for periods in excess of 3 months. • Senna singueana seedlings sucker readily and the suckering capacity increases with the plant (root) size. The optimum size which maximizes suckering has not, however, been established. Research is urgently required to determine this including the ascertainment of whether suckering potential is confined to the tape roots alone or spread evenly to the lateral roots.
ACKNOWLEDGEMENTS
- The author acknowledges with thanks the financial support from the Canadian International Development Research Centre (IDRC) and also wish to express his most sincere gratitude and appreciation to Sokoine University of Agriculture for her facilities and to all those people who in one way or another have contributed to the success of these studies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML