-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2015; 5(3): 51-56
doi:10.5923/j.plant.20150503.01
Root and Aerial Parts Flavonoids of 3 Iranian Carex L. (Cyperaceae) Species
Mitra Noori, Mehrana Jafari, Maryam Zakeri
Department of Biology, Faculty of Science, Arak University, Arak, Iran
Correspondence to: Mitra Noori, Department of Biology, Faculty of Science, Arak University, Arak, Iran.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Root and aerial parts flavonoids of 3 Carex L. species: Cariceae Pax. Tribe Cyperaideae Kostel. subfamily and Cyperaceae family [C. divisa Huds., Carex melanostachyaM. Bieb. ex Willd. (syn.: C. nutans Host) and C. stenophylla Wahlenb] from Iran were studied using two-dimentional paper chromatography (2-DPC) and thin layer chromatography (TLC). Carex plants are characterized by the production of stilbene derivatives and other bioactive polyphenols including lignans and flavonoids. By this reason they have attracted recent attention as potential food additives. Flavonoids are as one set of the polyphenolic compounds among secondary metabolites that are active principles of medicinal plants, exhibit pharmacological effects and contribute to human health. Collected plants were identified using available references and voucher samples were prepared as herbarium vouchers. Results showed all 3 studied species contain flavone C and C-/O-glycosides in their roots and aerial parts. Flavonoid sulphates were found in all of roots and aerial parts of the studied species with the exception of C. stenophylla root and aerial parts. Aglycones was not found in C. melanostachya aerial parts, where as other samples had. The studied taxa showed variety in their root and aerial parts flavonoids compounds. Rutin, Myricetin, Kaempferol, Loteulin, Narengenin, Apigenin, Morin, Rhamnetin and Chrysin were found in their root or aerial parts, while all of samples lacked Quercetin, Isorhamnetin, Tricin and Vitexin.
Keywords: Carex, Cariceae, Cyperaceae, Flavonoid compounds, Chromatography
Cite this paper: Mitra Noori, Mehrana Jafari, Maryam Zakeri, Root and Aerial Parts Flavonoids of 3 Iranian Carex L. (Cyperaceae) Species, International Journal of Plant Research, Vol. 5 No. 3, 2015, pp. 51-56. doi: 10.5923/j.plant.20150503.01.
Article Outline
1. Introduction
- Carex L. from Cariceae Pax. Tribe, Cyperaideae Kostel. Subfamily and Cyperaceae family includes sedges that dominate wetlands, pastures, prairies, tundra, and the herb layer of temperate forests [1]. More than 1800 species were recorded for the genus in the world that about 43 species are in Iran [2]. They have attracted recent attention as potential food additives because they contain high levels of bioactive polyphenols commonly found in plant foods. Despite there being as many as 2000 Carex species worldwide, only a few members including C. fedia, C. kobomugi, C. pumila, C. humilis, C. pendula, and C. distachya have been previously investigated for their phytochemical constituents. This is unfortunate because Carex plants are characterized by the production of stilbene derivatives and other bioactive polyphenols including lignans and flavonoids [3]. Feizbakhsh et al (2012) identified essential oils composition of C. pseudofoetida aerial parts from Iran [4]. Manhart (1986) reported foliar flavonoids of the North American members of the Carex section Laxiflorae [5]. Four metabolites, named carexanes I-L, have been isolated from the roots of C. distachya Desf, an herbaceous plant living in the Mediterranean Maquis, together with three known compounds, already isolated from the aerial part of the plant [6]. Also, they (2008) isolated 16 polyphenols, identified on the basis of spectroscopic data as 7 lignans, 4 phenylethanoids, 3 resveratrol derivatives, a monolignol, and a secoiridoid glucoside in C. distachya root. The species roots extract contained high quantities of polyphenols, most of them reported as constituents of edible plants, such as grape and olive, suggesting that the methanol root extract of this plant could be used as a source of natural antioxidants useful as potential food additives [1]. Seven compounds, which included two resveratrol oligomers and five flavonoids, were isolated from seeds of C. folliculata L. (northern long sedge), a forage prevalent in the northern United States. The flavonoids were isoorientin, luteolin, quercetin, 3-O-methylquercetin, and rutin [3]. Five flavonoids: tricin, tricin O-(erythro-b-guaiacylglyceryl) ether,apigenin-6-C-b-D-xylopyranosyl-8-C-b-D-glucopyranoside,apigenin-6-C-b-D-glucopyranosyl-8-C-b-D-xylopyranoside,luteolin-6-b-D-glucopyranosyl-8-C-b-D-xylopyranoside were reported from C. distachya Desf. fresh leaves [1]. Flavonoids as secondary metabolites are valuable and widely and effectively used in medicinal ingredients and chemosystematics [7]. The flavonoid work consists mostly of broad scale comparisons of aglycone distributions in a number of sedge genera to include some members of Carex. These comparisons proved to be of limited use in understanding phylogenetic relationships within the genus Carex. However, detailed flavonoid analyses of sections Laxiflorae and Acrocystis demonstrated the utility of flavonoid surveys in Carex at infrasectional levels and possibly at intersectional levels [8]. Catling et al (1989) used flavonoids for separating two hybrid C. lacustris × C. trichocarpa. Luteolin glycosides were found only in C. lacustris, whereas tricin glycosides were restricted to C. Trichocarpa [9]. Therefore, depth study of Carex L. medicinal ingredients and flavonoids can provided the basis for further development and utilization. In this study, root and aerial parts flavonoids of 3 Iranian Carex L. (Cyperaceae) species aqueous-ethanolic extracts are reported.
2. Materials and Methods
2.1. Collection of Plant Material and Preparation
- Mature fresh roots and aerial parts of 3 Carex species [C. divisa Huds., Carex melanostachya M. Bieb. ex Willd. (syn.: C. nutans Host) and C. stenophylla Wahlenb] were collected from Iran area during 2013 as described in Table 1. Plants identified using available references [2, 10, 11, 12]. Specimens of each sample were prepared for reference as herbarium vouchers that were deposited at the Arak University Herbarium. Root and aerial parts samples were separately air dried for detection and identification of flavonoids.
2.2. Extraction of the Plant Material
- For a comparative analysis of the flavonoids, small extracts of all the accessions were prepared by boiling 200 mg of powdered air dried root, and aerial parts material for 2 min in 5 ml of 70% EtOH. The mixture was cooled and left to extract for 24 h. The extract was then filtered, evaporated to dryness by rotary evaporation at 40°, and taken up in 2 ml of 80% MeOH for analysis by 2-Dimensional Paper Chromatography (2-D PC).
2.3. Flavonoid analysis by 2-Dimensional Paper Chromatography (2-DPC)
- For the detection of flavonoids, ca 20 μl of each of the small extracts was applied to the corner of a quarter sheet of Whatman No 1 chromatography paper as a concentrated spot (10 applications of 2μl). The chromatogram for each sample was developed in BAW (n-BuOH-HOAc-H2O=4:1:5; V/V; upper layer), 1st direction, and HOAc (=15% aqueous acetic acid), 2nd direction, with rutin (=quercetin 3-O-rutinoside) as a standard. After development, the chromatograms were viewed in longwave UV light (366 nm) and any dark absorbing and fluorescent spots were marked. Rf -values in BAW and 15% HOAc were calculated.
2.4. Methods of Identification of the Flavonoids
- After obtaining sufficient amounts of purified flavonoids, as in the case of the flavonoids from 3 Carex species roots and aerial parts, they were identified by means of UV spectroscopy using shift reagents to investigate the substitution patterns of the flavonoids and by acid hydrolysis to identify the aglycone and sugar moieties [13, 14]. Cochromatography with standards was also performed where possible. Flavonoid standards available for comparison during the study were Apigenin, Chrysin, Isorhamnetin, Kaempferol, Luteolin, Morine, Myricetin, Narengenin, Quercetin, Rhamnetin, Rutin, Tricine and Vitexin (all obtained commercially, Rutin from Merck, Apigenin and Luteolin from Sigma and the rest from Fluka).
2.5. Acid Hydrolysis and Identification of Flavonoid Aglycones
- A small amount of each purified flavonoid (ca 0.5 mg) was dissolved in 0.5 ml of 80% MeOH in a test tube. To this sample 2 ml of 2M HCl were added and the mixture was heated in a water bath at 100°C for 0.5 h. The solution was cooled, 2 ml of EtOAc were added and thoroughly mixed with the aqueous layer using a whirley mixer. The upper EtOAc layer was removed with a pipette, evaporated to dryness, dissolved in 0.5 ml of MeOH and applied as spots on thin layer chromatograms (cellulose). The TLC plates were run in three solvents alongside standards to identify the aglycone moiety [15].
3. Results
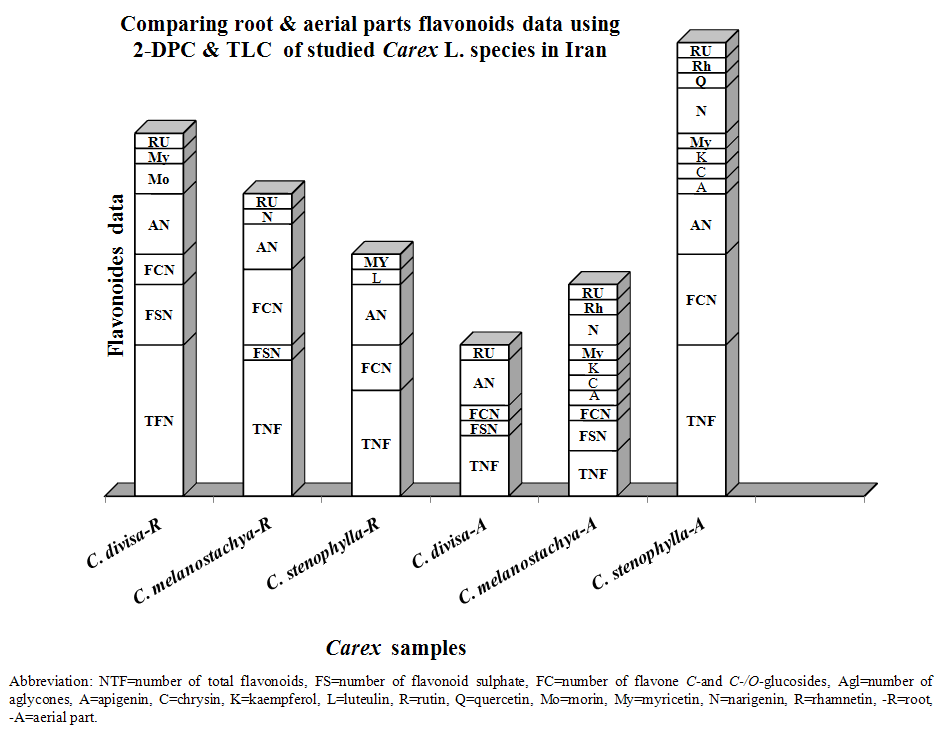
- All studied Carex species contained flavonoid compounds in their roots and aerial parts. Their flavonoid profiles showed a wide variety between the species. Data in Tables 1 and 2 show the sampling and also 2-dimentional paper and thin layer chromatographical data of 3 studied Carex species from Iran. Figure 1 shows stacked column with a 3-D visual effect histogram for comparing root and aerial parts flavonoids data (number of flavonoid sulphate, number of flavone C-and C-/O-glucosides, number of aglycones and occurrence of Apigenin, Chrysin, Isorhamnetin, Kaempferol, Luteolin, Morine, Myricetin, Narengenin, Quercetin, Rhamnetin, Rutin, Tricine and Vitexin) in the species. The studied taxa showed variety in their root and aerial parts flavonoids compounds. As Table 1 and Figure 1 show all 3 studied species contain flavone C and C-/O-glycosides in their roots and aerial parts. Flavonoid sulphates were found in all of roots and aerial parts of the studied species with the exception of C. stenophylla root and aerial parts. Aglycones was not found in C. melanostachya aerial parts, where as other samples had. Rutin, Myricetin, Kaempferol, Loteulin, Narengenin, Apigenin, Morin, Rhamnetin and Chrysin were found in their root or aerial parts, while all of samples lacked Isorhamnetin, Tricin and Vitexin. C. divisa root and C. stenophylla aerial parts had the most flavonoids number. At least flavononoid number was observed in aerial parts of C. melanostachya.
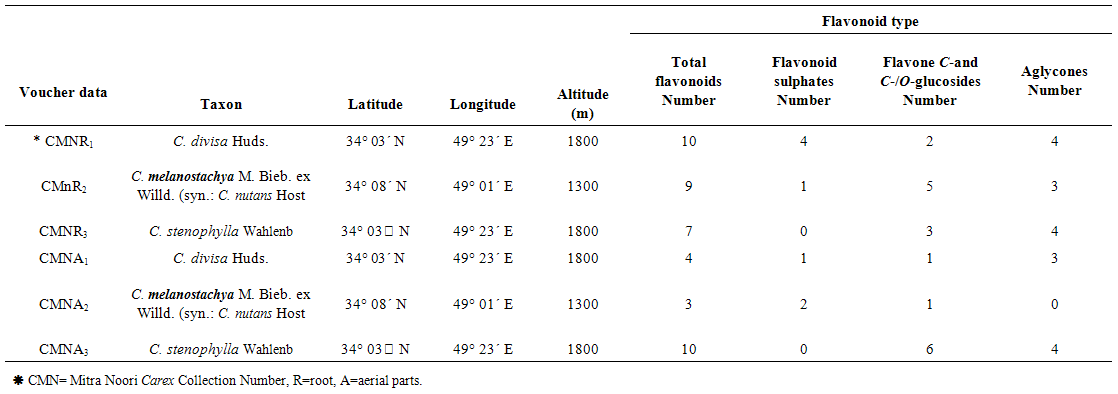 | Table 1. Collection information and 2-Dimentional Paper Chromatography flavnoids data of 3 studied Carex species roots and aerial parts from Iran |
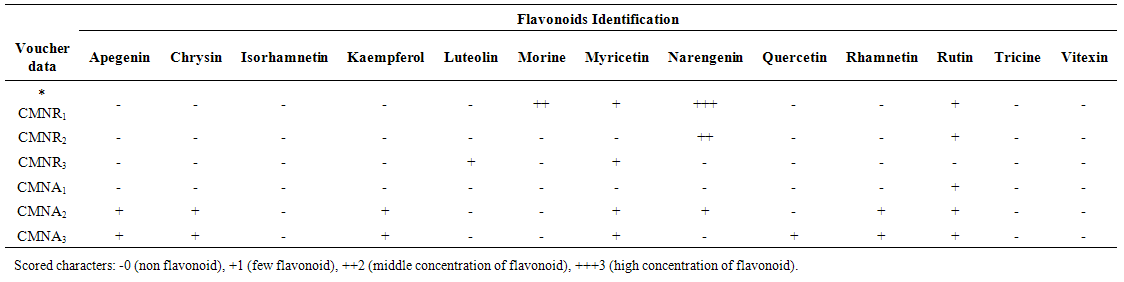 | Table 2. Thin Layer Chromatography data of 3 studied Carex species roots and aerial parts from Iran |
4. Discussion and Conclusions
- Flavonoids are as one set of the polyphenolic compounds among secondary metabolites in different organs of plants that are popular compounds for chemotaxonomic surveys of plant genera and families [16]. Also many flavonoids are active principles of medicinal plants, exhibit pharmacological effects and contribute to human health [16-18]. Several studies indicated that flavonoids occurred in various species of Cyperaceae [19-21]. The presence of the characteristic leaf flavonoids (glycoflavones, tricin) of the grasses in this family shows that the Cyperaceae and the Gramineae are more closely linked chemically than a previous study of their inflorescence pigments suggested [22]. As Harborne et al (1982) studies on 92 Australian Cyperus species showed phytochemical studies of the Cyperaceae have been extremely useful in clarifying systematic relationships within the family members and flavonoids may be useful taxonomic markers within the family [22, 23]. These results show that phenolic patterns appear to be more useful for studying relationships within relatively narrow taxonomic limits, e. g. at the species and genus level [16]. Noori (2014) compared 10 populations root and leaf flavonoids profiles of 5 Scirpus species from Markazi Province, Iran for introducing chemotypes. Her results showed all of studied Scirpus populations contain vitexin, luteolin, rutin and rhamnetin in their aerial parts and roots. Also morin and tricin are two separator phytochemical characters for studied samples [24].As we know Cyperaceae flavonoids are very important for their different potential clinical applications such as their toxicity, antidiarrhoeal, antibacterial, antiflogestic, tonic and stimultant effects [25-27]. S. lacustris is used as local medicinal plant in Canada [28], its stem known as antibacterial drug and is effective on E. coli [29]. Studying root flavonoids of 5 Scirpus L. species from Cyperaceae (S. holoschenus L., S. lacustris L., S. littoralis Kuntze, S. maritimus L. and S. multicaule) from different parts of Markazi Province, Iran area showed all of studied taxa contain flavonoid sulphates, flavone C and C-/O-glycosides and aglycones in their roots, while Rutin, Myricetin and Vitexin were just found in S. maritimus. Also presence of Morin, Tricin and Loteulin in all of the species roots with the exception of S. maritimus are more valuable tools for taxa separation. Kaempferol was found in S. lacustris and S. littoralis species, where as others lack [30]. Plants of the Carex genus have attracted recent attention as potential food additives because they contain high levels of bioactive polyphenols commonly found in plant foods [3]. As Fiorentino et al (2008) studies showed high quantities of polyphenols in C. distachya species root methanol extract that could be used as a source of natural antioxidants useful as potential food additives [1]. Li et al (2009) isolated five flavonoids (isoorientin, luteolin, quercetin, 3-O-methylquercetin, and rutin) from C. folliculate seeds [3]. Also Manhart (1986) reported foliar flavonoids of the North American members of the Carex section Laxiflorae [5].Our results showed existing flavonoid sulphates in all of roots and aerial parts of the studied species with the exception of C. stenophylla root and aerial parts. Aglycones was not found in C. melanostachya aerial parts, where as other samples had. The studied taxa showed variety in their root and aerial parts flavonoids compounds. Rutin, Myricetin, Kaempferol, Loteulin, Narengenin, Apigenin, Morin, Rhamnetin and Chrysin were found in their root or aerial parts, while all of samples lacked Quercetin, Isorhamnetin, Tricin and Vitexin (Tables 1 and 2, Figure 1)). Based on these results it is concluded that the quantities and presence of important metabolites such as flavonoids depend on the various parts of the plant used. Therefore, depth study of Carex L. medicinal ingredients and flavonoids can provided the basis for further development and utilization.
References
| [1] | FIORENTINO A, RICCI A, D'ABROSCA B, PACIFICO S, GOLINO A, LETIZIA M, PICCOLELLA S, MONACO P. 2008. Potential food additives from Carex distachya roots: identification and in vitro antioxidant properties. J Agric Food Chem. 2008 Sep 10; 56 (17): 8218-25. |
| [2] | GHAHREMAN A. 1994. Plant Systematics, Cormophytes of Iran; Markaze Nashr-e Daneshgahi, Tehran-Iran, No. 739, Vol. 4, 332-365. |
| [3] | LI L, HENRY GE, SEERAM NP. 2009 Aug, Identification and bioactivities of resveratrol oligomers and flavonoids from Carex folliculata seeds. J Agric Food Chem. 26, 57 (16): 7282-7282. |
| [4] | FEIZBAKHSH A, AGHASSI A, NAEEMY A. 2012. Composition of the essential oil of Carex pseudofoetida, Chemistry of Natural Compounds, 48 (6): 1087-1088. |
| [5] | MANHART JR. 1986. Foliar flavonoids of the North American members of the Carex section Laxiflorae (Cyperaceae). Biochem. Syst. Ecol. 14, 85-90. |
| [6] | FIORENTINO A, D'ABROSCA B, PACIFICO S, NATALE A, MONACO P. 2006. Structures of bioactive carexanes from the roots of Carex distachya Desf. Phytochemistry. 67 (10): 971-977. |
| [7] | NOORI M. 2002. Characterization of the Iranian species of Sophora and Ammodendron (Leguminosae; Sophoreae), PhD Thesis, University of London and Royal Botanic Gardens, Kew, UK. |
| [8] | MANHART JR. 1990. Chemotaxonomy of the genus Carex (Cyperaceae), Canadian Journal of Botany, 68 (7): 1457-1461. |
| [9] | CATLING PM, REZNICEK AA, DENFORD K. 1989. Carex lacustris × C. trichocarpa (Cyperaceae), a new natural hybrid, Canadian Journal of Botany, 67 (3): 790-795. |
| [10] | MOBAYEN S. Iran Vegetation (Vascular Plant Flora), Tehran University Publications, 1979, no. 1500/2253, Vol. 1: 209-244. |
| [11] | GHAHREMAN A. 1979-2009. Flore de l'Iran, A join project by the Research Institute of Forests and rangelands (Iran) and Tehran University, Published by RIFR, Ministry of Reconstruction Jahad, Volumes 1-24. |
| [12] | LUNKAI D, SONGZUM L, ZHANG S, TANG Y, KOYAMA T, TUCKER GC, SIMPSON DA, NOLITE HJ, STRONG MT, BRUHL JJ, WILSON KL, MUASYA AM. 2010. Flora of China.Cyperaceae, 23: 164-461. |
| [13] | MABRY TJ, MARKHAM KR, THOMAS MB. 1970. The Systematic Identification of Flavonoids, Springer Verlag, Berlin. |
| [14] | MARKHAM KR. 1982. Techniques of Flavonoid Identification, Academic Press, London. |
| [15] | HARBORNE JB. 1998. Phytochemistry Methods, 3rd ed. Chapman and Hall, London. |
| [16] | HARBORNE JB. 1994 The Flavonoids: Advance in research since 1986, Chapman and Hall, New York. |
| [17] | MOORE MO, GIANNASI DE. 1994, Foliar flavonoids of. Eastern North American vitis (vitaceae) North of Mexico. Plant systematics and evolution, 193 (1-4): 21-36. |
| [18] | NOORI M, CHEHREGHANI A, KAVEH M. 2009. Flavonoids of 17 species of Euphorbia, (Euphorbiaceae) in Iran, Toxicological and Environmental Chemistry, 91 (3): 409-418. |
| [19] | GLENNIE W, HARBORNE JB. 1971. Flavone and flavonol 5-glucosides, Phytochem. 10, 1379. |
| [20] | WILLIAMS CA, HARBORNE JB. 1977. Flavonoid chemistry and plant geography in the Cyperaceae, Biochemical Systematics and Ecology, 5 (1): 45–51 (available online 28 Jan. 2003). |
| [21] | NOORI M. 2012. Flavonoids in some Iranian angiosperms, In: Phytochemicals-A Global Perspective of Their Role in Nutrition and Health, A. Venketeshwer Rao, (Eds.). INTECH Publisher, pp: 151-166, http://www.intechopen.com. |
| [22] | HARBORNE JB. 1971. Distribution and taxonomic significance of flavonoids in the leaves of the Cyperaceae, Phytochemistry, 10 (7): 1569–1574. |
| [23] | HARBORNE JB, WILLIAMS CA. WILSON KL. 1982. Flavonoids in leaves and inflorescences of australian cyperaceae, Phytochemistry, 24 (4): 751-766. |
| [24] | NOORI M. 2014. Introducing Scirpus L. chemotypes in Markazi Province, Iran, OWSD Fifth General Assembly and International Conference, Cuernavaca, Mexico 17-20 September 2014. |
| [25] | CRONQUIST A. 1981. An integrated system of classification of flowering Plants. Columbia Univ. Press, New York. |
| [26] | MIRHEIDAR H. 1993. Maaref-e-Giahi. Vol. 4, Islamic Culture Office Publication, Iran, Pages: 303. |
| [27] | JOUNGDUK J, HONG KC. 2010. Systematic Rearrangement of Korean Scirpus L. (Cyperaceae) as Inferred from Nuclear ITS and Chloroplast rbcl Sequences, Journal of Plant Biology, 53 (3): 222- 232. |
| [28] | HEBDA J. 1981. Use of plants for food and medicine by Native Peoples of eastern Canada, Canadian Journal of Botany, 59 (11): 2189- 2325. |
| [29] | VILLARS MT, DELVIGNE. 2001. Estuarine processes. Literature Review. ECO3B_Estuarine_Processes_Feb_2001_FinalReport.pdf. |
| [30] | NOORI M, DEHSHIRI MM, MEHRDOST N.. 2012. Root flavonoids of some Iranian Scirpus L. (Cyperaceae) members, International Journal of Botany, 8 (3): 165-169. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML