-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2015; 5(2): 42-49
doi:10.5923/j.plant.20150502.03
Comparative Studies of Leaf, Gall and Bark Flavonoids in Collected Quercus brantii Lindl. (Fagaceae) from Lorestan Province, Iran
Mitra Noori1, Mahdi Talebi1, Tahere Ahmadi2
1Department of Biology, Faculty of Science, Arak University, Arak, Iran
2MSc student of Department of Biology, Faculty of Science, Arak University, Arak, Iran
Correspondence to: Mitra Noori, Department of Biology, Faculty of Science, Arak University, Arak, Iran.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Many flavonoids are active principles of medicinal plants, exhibit pharmacological effects and contribute to human health. Also they are taxonomically important for chemosystematic studies by the reason universal presence in vascular plants. In this study leaf, gall and bark flavonoids of nine populations of Quercus brantii L. varieties (Q. brantii var. belangeri and Q. brantii var. persica) are compared and reported for first time. Aqueous-ethanolic extracts of collected plant material from Lorestan Prvince aera, Iran were examined to practice flavonoid detection, isolation and identification by 2-dimensional paper chromatography (2-DPC), thin layer chromatography (TLC), UV spectroscopy and available references. Voucher specimens were prepared for reference as herbarium voucher. Results showed all of examined taxa have flavonoid sulphate, flavon C & C-/O glycosides and aglycons in their leaf, gall and bark. Apigenin, kaempferol, myricetin, naringenin, quercetin and rutin were nearly found in all of studied taxa parts. Studied samples leaf and bark had vitexin while their galls lacked.
Keywords: Quercus, Fagaceae, Flavonoids, Leaf, Bark, Gall, Iran
Cite this paper: Mitra Noori, Mahdi Talebi, Tahere Ahmadi, Comparative Studies of Leaf, Gall and Bark Flavonoids in Collected Quercus brantii Lindl. (Fagaceae) from Lorestan Province, Iran, International Journal of Plant Research, Vol. 5 No. 2, 2015, pp. 42-49. doi: 10.5923/j.plant.20150502.03.
Article Outline
1. Introduction
- The genus Quercus L. (oak) from Fagaceae family consists of more than 400 species over the worlds. It has historically been an important source of fuel, fodder and building materials throughout their ranges. Other products include tannins and dyes, oak bark and leaves were often used for tanning leather [1, 2]. Genus Quercus has a problematic taxonomy [3] because of its immense size and wide distribution [4], heterophylly, widespread hybridization between the inferageneric taxa and changes in morphological features [5, 6]. Therefore, several taxonomic groups within the genus are noted for their complex patterns of variation, which causes difficulties for identification of taxa at the species level [7]. For these reasons there are many discussions about species numbers in this genus and it is estimated that Quercus comprising more than 400 species over the worlds [8, 9], but modern taxonomic treatments will probably reduce that number [10]. Quercus species are the most spread trees in the Mediterranean forests [11], which occupies vast territories of the Northern Hemisphere in North America, Europe and Asia [12-15]. They are the most diverse species in Iran forests [16]. Several oak species grow abundantly in the Zagros, Arasbaran and Hyrcanian forests displaying remarkable morphological variation. All oak species of Iran belong to the subgenus Quercus and two sections: Quercus and Cerris [17]. Q. brantii Lindl. is one of the Quercus subgenus Quercus, section Cerris Spach [18]. This species covering more than 50% of the Zagros forest area and is the most important tree species of this region growing at 1000-2000 m altitudes [19]. Oaks are important source of wood and fiber. Their fruits has great characteristics such as medical and nutritional. Oak akenes include starch, protein, oil and tannins. They are useful in treatment of anemia, diarrhea, etc, and the other one application is livestock feeding [20]. There are many studies about pharmacognostic properties of different organs of Quercus species special gall. Shrestha et al (2014) showed pharmacognotic effects of Q. infectoria Olivier insect gall in their studies. Results showed the presence of phenols, flavonoids, steroids, triterpenes, tannins, saponins and alkaloids. Identification of these medicinal plant materials can be useful for future plant drug applications. The main constituents found in the galls of Q. infectoria are tannin (50-70%) and small amount of free gallic acid and ellagic acid [21]. Scherbath (2002) found that oaks contain about 25-28 chemical compounds. These include tannic acid, egallic acid, ellagic acid, monoterpens, pcoumarin, vanillic acid, tolune and kaempferol [22]. Four species of oak (Q. aegilop, Q. infectoria, Q. libani and Q. Marcantherea) grown in the Iraqi Kurdistan forest contain secondary products such as polyphenolic compounds; tannins are ellagic acids which are considered to be a great importance in medicinal, pharmaceutical, antimicrobial and anti disease [23]. Ghafour et al (2010) determined some chemical constitutes of acorn, pericarp and cupules in the 3 oak species (Q. aegilops, Q. infectoria and Q. libani) from the Mountain Oak Forest of Sulaimani Governorate. Results indicated the existence of significant differences in total phenols and total tannins [24]. Kim et al (2012) comprised phenolic compounds content in five Korean indeciduous Quercus species. These species have been used in Korean folk medicine, having effects on various diseases such as dysentery, diarrhea, hemorrhagia and dermatitis. They found that phenolic compounds concentration in leaf was higher than shoot and Q. salicine Blume leaf had the highest concentration of total phenolic compounds among the studied taxa [25]. Nebigil (2011) studied on antioxidative, and antimicrobial properties of Q. brantii L. (Q.brantii.) seed extract and determined its phenolic profile using High Performance Liquid Chromatography (HPLC). He found that isolated methyl gallate and ethyl acetate fractions could be considered as powerful antimicrobial agents, and at the same time efficacious antioxidants [26]. Flavonoids constitute are the largest group of plant phenols and naturally generating phenolic compounds. They have the basic skeleton of diphenylpropanes [27]. Flavonoids are as one set of the polyphenolic compounds among secondary metabolites in different organs of plants that possess a wide range of biological activities. In addition they have medicinal and pharmacological effects [28-30]. Also they are popular characters for chemosystematic studies because: the almost universal presence of flavonoids in vascular plants; 2. Their structural diversity; 3. The fact that each species usually contains several flavonoids; 4. The chemical stability of many flavonoids in dried plant material enabling herbarium material to be used; 5. Flavonoid profiles using different chromatographic techniques are easily obtained. 6. Flavonoids are reasonably easy to identify using published UV spectra data and available standards; 7. Flavonoids often show correlations with existing classifications at the family, genus and species level, and support revisions of existing classifications at the family, genus and species level [31]. Plant phenolic patterns appear to be more useful for studying relationships within relatively narrow taxonomic limits, e. g. at the species and genus level [30, 32, 33]. Noori (2014) compared 10 population root and leaf flavonoids profiles of 5 Scirpus species from Markazi Province, Iran for introducing chemotypes [34]. Meng et al (2001) studied on the effect of five flavonoid compounds isolated from Q. dentata Thunb on superoxide generation in human neutrophils and phosphorylation of neutrophil proteins. They found that the superoxide generation induced by phorbol 12-myristate 13-acetate (PMA) was suppressed by all yellow compounds [35]. Zhout et al (2001) isolated four acylated flavonoid glycosides from the leaves of Q. dentata Thunb. They were kaempferol 3-O-[2"-O-(trans-p-coumaroyl)-6"-O -acetyl]-β-D-glucopyranoside, kaernferol 3-O-[2",6"-di-O- (trans-p-coumaroyl)-3",4"-di-O-acetyl]-β-D-glucopyranoside, kaempferol 3-O-[2",6"-di-O-(trans-p-coumaroyl)]-β-D- glucopyranoside and kaempferol 3-O-[6"-O-(trans-p- coumaroyl)]-β-D-glucopyranoside [36]. In this study leaf, gall and bark flavonoids of nine populations of Q. brantii L. varieties (Q. brantii var. belangeri and Q. brantii var. persica) aqueous ethanolic extracts are reported for first time in Iran.
2. Materials and Methods
2.1. Collection of Plant Material and Preparation
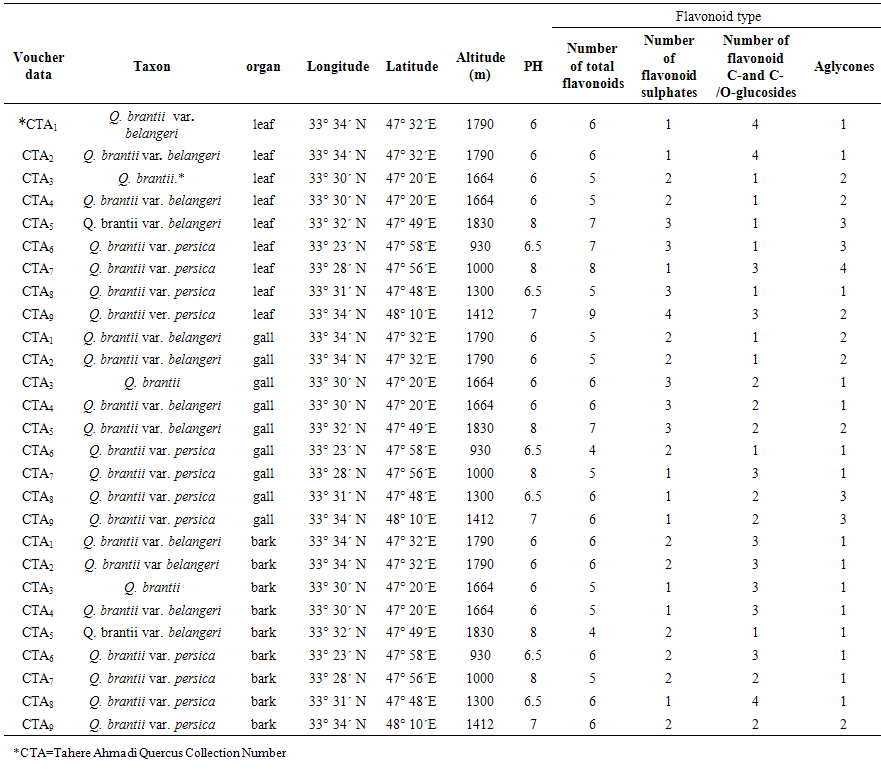
- Leaf, gall and bark of nine populations of Quercus brantii L. varieties (Q. brantii var. belangeri and Q. brantii var. persica) were collected from Lorestan Province, Iran area during 2013 as described in Table 1. Plants identified using available references [3, 4, 6, 37, 38]. Specimens of each sample were prepared for reference as herbarium vouchers that were deposited at the Arak University Herbarium. Leaf air dried, gall and bark samples were separately prepared for detection and identification of their flavonoids.
2.2. Extraction of the Plant Material
- For a comparative analysis of the flavonoids, small extracts of all the accessions were prepared by boiling 200 mg of powdered each of materials for 2 min in 5 ml of 70% EtOH. The mixture was cooled and left to extract for 24 h. The extract was then filtered, evaporated to dryness by rotary evaporation at 40, and taken up in 2 ml of 80% MeOH for analysis by 2-Dimensional Paper Chromatography (2-D PC).
2.3. Flavonoid Analysis by 2-Dimensional Paper Chromatography (2-DPC)
- For the detection of flavonoids, ca 20 μl of each of the small extracts was applied to the corner of a quarter sheet of Whatman No 1 chromatography paper as a concentrated spot (10 applications of 2μl). The chromatogram for each sample was developed in BAW (n-BuOH-HOAc-H2O=4:1:5; V/V; upper layer), 1st direction, and HOAc (=15% aqueous acetic acid), 2nd direction, with rutin (=quercetin 3-O-rutinoside) as a standard. After development, the chromatograms were viewed in longwave UV light (366 nm) and any dark absorbing and fluorescent spots were marked. Rf -values in BAW and 15% HOAc were calculated.
2.4. Methods of Identification of the Flavonoids
- After obtaining sufficient amounts of purified flavonoids, as in the case of the flavonoids from nine populations of Q. brantii varieties (Q. brantii var. belangeri and Q. brantii var. persica), they were identified by means of UV spectroscopy using shift reagents to investigate the substitution patterns of the flavonoids [39, 40] and by acid hydrolysis to identify the aglycone and sugar moieties. Cochromatography with standards was also performed where possible. Flavonoid standards available for comparison during the study were Apigenin, Chrysin, Isorhamnetin, Kaempferol, Luteolin, Morine, Myricetin, Narengenin, Quercetin, Rhamnetin, Rutin, Tricine and Vitexin (all obtained commercially, Rutin from Merck, Apigenin and Luteolin from Sigma and the rest from Fluka).
2.5. Acid Hydrolysis and Identification of Flavonoid Aglycones
- A small amount of each purified flavonoid (ca 0.5 mg) was dissolved in 0.5 ml of 80% MeOH in a test tube. To this sample 2 ml of 2M HCl was added and the mixture was heated in a water bath at 100C for 0.5 h. The solution was cooled, 2 ml of EtOAc was added and thoroughly mixed with the aqueous layer using a whirley mixer. The upper EtOAc layer was removed with a pipette, evaporated to dryness, dissolved in 0.5 ml of MeOH and applied as spots on thin layer chromatograms (cellulose). The TLC plates were run in three solvents alongside standards to identify the aglycone moiety [41].
3. Results
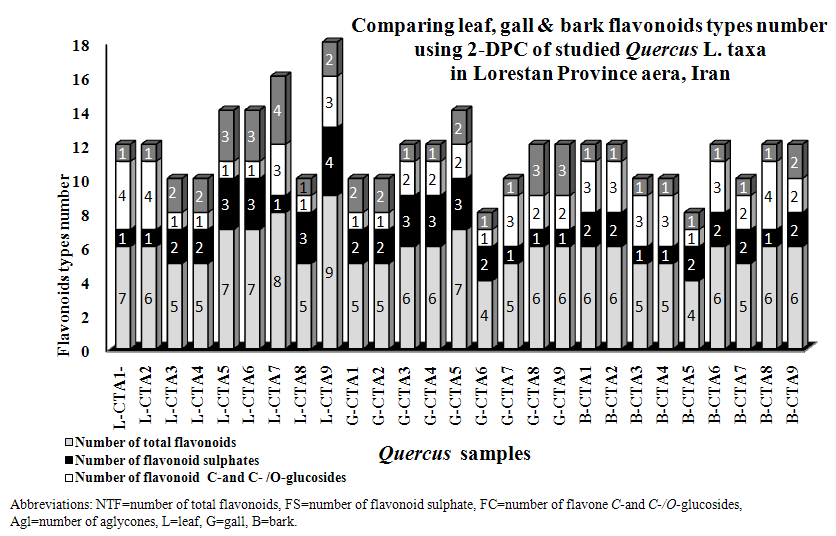
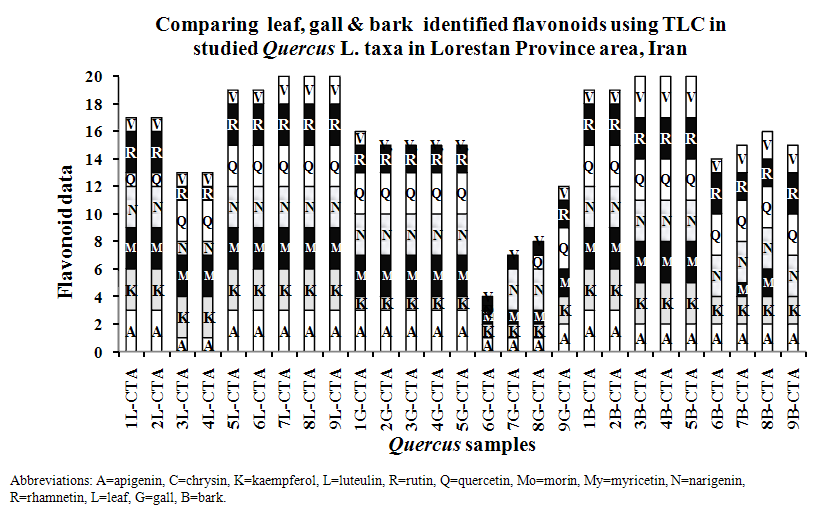
- All of studied Quercus samples contained flavonoid compounds in their leaf, gall and bark. Their flavonoids profiles showed a wide variety between the samples. Data in Table 1 and 2 show the sampling and also 2-dimentional paper and thin layer chromatographical data of nine populations of Q. brantii varieties from west of Iran. Figure 1 and 2 show stacked column with a 3-D visual effect histogram for comparing leaf, gall and bark flavonoids data (number of flavonoid sulphate, number of flavone C-and C-/O-glucosides, number of aglycones and occurrence of Apigenin, Kaempferol, Myricetin, Narengenin, Quercetin, Rutin and Vitexin) in the studied samples. As Table 1 and Figure 1 show all 9 studied species contain flavonoid sulphates, flavone C and C-/O-glycosides and aglycons in their leaf, gall and bark. As Table 2 and Figure 2 show the most flavonoid compounds variety was found in CTA7, CTA8 and CTA9 leaves and also in CTA3, CTA4 and CTA5 barks. Apigenin and Kaempferol were found in all of nine Q. brantii varieties populations leaf, gall and bark. All of studied samples had Myricetin with the exception of CTA6 gall and CTA9 bark. Quercetin was found in all of leaf, gall and bark of the studied taxa with the exception of CTA6 and CTA7 galls. All of studied taxa leaf, gall and bark had Narengenin but CTA6 and CTA9 galls lacked this flavonoid. CTA6, CTA7 and CTA8 galls had not Rutin while other samples had. Vitexin was found in all of studied Quercus samples leaf and bark. All of studied Quercus samples gall lacked Vitexin with the exception of CTA1 and CTA9 galls.
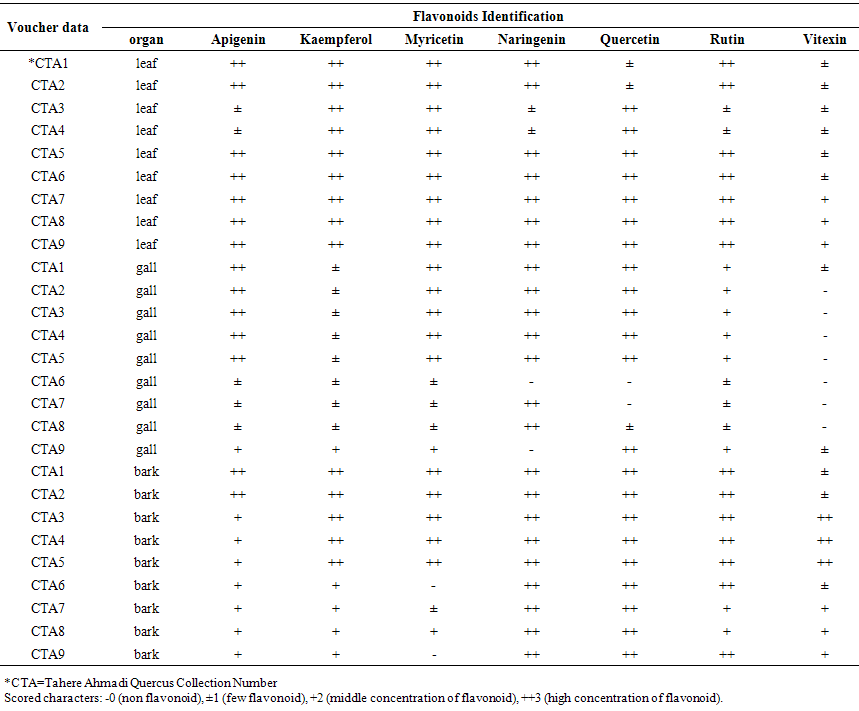 | Table 2. Thin Layer Chromatography data of 9 studied populations of Quercus brantii L. varieties (Q. brantii var. belangeri and Q. brantii var. persica) leaf, gall and bark from Iran |
4. Discussion and Conclusions
- It is known that oaks in addition being important for their wood and fiber, they are important in medicine and nutrition. Many studies showed existing phenolic and flavonoids compounds in different Quercus species organs special in gall and fruit [21-27]. Existing flavonoids in oaks was reported by some researchers but identification of flavonoids kinds in Iranian oaks was reported for first time in this study. All of studied Quercus samples contained flavonoid compounds in their leaf, gall and bark that the most flavonoid compounds variety was found in CTA7, CTA8 and CTA9 leaves and also in CTA3, CTA4 and CTA5 barks (Tables 1 and 2, Figures 1 and 2). These results show a wide variety between the samples in the studied samples flavonoids profiles. Ghafour et al (2010) studies on 3 oak species acorn, pericarp and cupules showed the existence of significant differences in total phenols and total tannins [24]. Our study results showed existing of Apigenin, Kaempferol, Myricetin, Narengenin, Quercetin, Rutin and Vitexin in studied taxa. Osterc et al (2007) and Pulcini (2006) found these named flavonoids in Castanea taxa. Also Laposi et al (2008) reported flavonid compounds from Fagus sylvatica [42-44]. These results can extremely be useful in clarifying systematic relationships within the family as Harborne (1994) showed among secondary metabolites in different organs of plants, flavonoids are popular compounds for chemotaxonomic surveys of plant genera and families [32] in addition their role as active principles of medicinal plants, exhibition pharmacological effects and contribution to human health [30, 32, 33]. The phenolic patterns appear to be more useful for studying relationships within relatively narrow taxonomic limits, e. g. at the species and genus level as Williams et al (1986) used the character for 255 species of the family Iridaceae [45] and Noori (2014) studies showed in Scirpus populations [34]. Q. brantii galls are used in tanning leather and also used in traditional medicine with other Quercus organs. Occurrences of flavonoid compounds in studied organs of Q. brantii varieties are good character by the reason flavonoids are active principles of nutritional and medicinal plants. Studies on their flavonoids antioxidatnt, antifungal and antibacterial activities can help to better usage of them and also improvement of drugs and foods quality. Nebigil (2011) studied on antioxidative, and antimicrobial properties of Q. brantii seed extract [26]. Meng et al (2001) studied on the effect of five flavonoid compounds isolated from Q. dentata Thunb on superoxide generation in human neutrophils and phosphorylation of neutrophil proteins [35]. Further work is needed using high performance liquid chromatography with diode array detection, atmospheric pressure chemical ionization liquid chromatography-mass spectroscopy to evaluate all flavonoid profiles in studied and other Quercus species. It is believed that Quercus species can be separated from each other according to their flavonoid pattern and also the pattern can help to more their usages.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML