-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2014; 4(2): 56-61
doi:10.5923/j.plant.20140402.03
Profile of Antioxidants and Scavenger Enzymes during Different Developmental Stages in Vigna radiata (L.) Wilczek (Mungbean) under Natural Environmental Conditions
Piku Sen1, Anulipi Aich1, Anandita Pal2, Supatra Sen3, Debasish Pal3
1Govt. College of Engineering & Leather Technology, Calcutta, Post Code: 700098, W.B., India
2Integrated M.Sc. Biotechnology, St. Xaviers’ College, Calcutta, Post Code: 700016, W.B., India
3School of Life Science, Uluberia College, Uluberia, Howrah, Post Code: 711315, W.B., India
Correspondence to: Debasish Pal, School of Life Science, Uluberia College, Uluberia, Howrah, Post Code: 711315, W.B., India.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Leaf samples of Vignaradiata (L.) Wilczek (Mungbean) were collected at different developmental stages viz. 6, 25, 45, 60 and 75 days, grown under natural environmental conditions and were analyzed for studying the profile of antioxidants – carotene, xanthophyll and ascorbic acid along with scavenger enzymes – catalase, peroxidase and ascorbic acid oxidase. This paper discusses the changes in the enzymatic and non-enzymatic antioxidant content in correlation to the physiological and metabolic status of the crop in different growth stages. Our work indicates that 25 to 45 days after sowing (DAS) is the favorable period (active growth) and 60 DAS is the stressful stage (comparable to senescence) in the Vignaradiata life cycle in terms of antioxidant profile and scavenging enzymes. The findings will address future problems related to stress physiology and senescence of this particular legume. It may lead to further studies for delaying senescence of this particular crop using Recombinant DNA technology.
Keywords: Antioxidant, Scavenging enzymes, Mungbean, Carotene, Xanthophyll, Ascorbic acid, Catalase, Peroxidase, Ascorbic acid oxidase
Cite this paper: Piku Sen, Anulipi Aich, Anandita Pal, Supatra Sen, Debasish Pal, Profile of Antioxidants and Scavenger Enzymes during Different Developmental Stages in Vigna radiata (L.) Wilczek (Mungbean) under Natural Environmental Conditions, International Journal of Plant Research, Vol. 4 No. 2, 2014, pp. 56-61. doi: 10.5923/j.plant.20140402.03.
Article Outline
1. Introduction
- Plants possess enzymatic and non-enzymatic antioxidative defense systems in the organelles of their cells. Catalase, peroxidase, and Superoxide dismutase, enzymes in the ascorbate-glutathione cycle, such as ascorbate peroxidase, monodehydroascorbate reductase, dehydroascorbate reductase, and glutathione reductase are examples of major antioxidative enzymes [1-2]. In this study out of these, three marker enzymes catalase, peroxidase and ascorbic acid oxidase were selected as indices of the physiological and metabolic status of different developmental stages in legume Vigna radiata (L.) Wilczek (Mungbean) under natural environmental conditions. The major non-enzymatic antioxidants are ascorbate (vitamin C), carotenoid (carotene and xanthophylls), glutathione, α-tocopherol (vitamin E), β-carotene, and flavonoids; these antioxidants are distributed chiefly in chloroplasts, but also occur in other cellular compartments, such as mitochondria and peroxisomes [1]. Of these, two non-enzymatic antioxidants viz. carotenoid (carotene and xanthophylls) and ascorbic acid were selected as work parameters. Mungbean is an important traditional leguminous crop of India characterized by a relatively high content of protein and is a short summer season crop. Abundant information is available on legume plant responses under different kinds of imposed abiotic stresses [3-4]. Information is relatively sparse on activities of scavenging enzymes and antioxidants at the various developmental stages in legumes under natural environmental conditions, which is why this particular research work was specifically carried out. This research was undertaken to study the response of the mungbean plant under natural tropical conditions of Kolkata, India in different developmental stages. The antioxidant and enzyme profile in the different stages could serve as an indicator of the physiological status of the plant under natural environmental conditions which in turn could indicate the most optimum and/or stressful developmental stage for further studies in future. Since many different agriculturally important traits are affected by senescence, which in turn, is strongly affected by the scavenging enzymes and antioxidants under study, this work related to antioxidant and scavenger enzyme profile in different developmental stages of mungbean (an important crop plant) may contribute to address this particular aspect.
2. Materials & Methods
- Vigna radiata (L.) Wilczek (Mungbean), chosen for the experimental work is an excellent and cheap source of high quality protein. Mungbean is consumed as whole grain as well as pulse in a variety of ways. It is also a green manuring crop. Being a leguminous crop, it has the capacity to fix atmospheric nitrogen. The soil was prepared by mixing farmyard manure in accordance with normal agronomic practices to avoid any changes in edaphic conditions. The clay pots of 14 inch diameter were filled with 8 kg of soil per pot. The sieve garden soil and sand were mixed in the ratio of 3:1. After preparation of pots, they were arranged in rows in the experimental garden at Govt. College of Leather Technology, Salt Lake, Kolkata (22.340 North and 88.240 East), West Bengal, India. The experiments were conducted in the summer months of March-June 2013. The Meterological Data were collected from Regional Meterological Centre, Alipore, Kolkata. (Table 1).Certified seeds of Vigna radiata (L.) Wilczek variety SML668 was obtained from National Seeds Corporation Ltd., Sector IV & V, Block AQ, Kolkata. Healthy surface sterilized [5] seeds of mungbean were selected and soaked in distilled water for 6 hours to initiate better germination and for early seedling development. These pots were placed in the open air of the wire netting enclosure to save seeds and young seedlings from birds. After complete germination, number of seedlings per pot was reduced to 4 by thinning to ensure that 4 plants were of more or less equal size and vigour. All pots were watered uniformly throughout the experimental period in order to maintain constant soil moisture. Healthy leaf samples were collected at 6, 25, 45, 60 and 75 days after sowing (DAS) and cleaned with sterile distilled water before weighing. All leaf samples were homogenized in mortar and pestle with respective buffer for estimation of different enzymatic and non enzymatic antioxidants.Non-enzymatic antioxidants viz. Carotene and xanthophyll (carotenoid) were estimated according to the method of Davies [6] and Ascorbic acid content according to Mitsui and Ohta [7]. Ascorbic acid oxidase activity was assayed according to Oberbacher and Vines [8]; scavenging enzymes viz. Catalase enzyme was assayed according to method of Gasper and Lacoppe [9] and Peroxidase according to Chance and Maehly [10] with slight modifications. Protein content was estimated according to Lowry [11]. The data obtained from three replications were statistically analyzed. Standard Deviation (SD) and Critical Difference (CD) values of different developmental stages at 5% were calculated from the analysis of variance (ANOVA) table. SD of the data is shown in the graphs and the calculated CD value is also provided.
3. Results and Discussion
- Meterological data spanning the months of March-June (summer season) 2013 were collected from the Regional Meterological Centre, Alipore, Kolkata. Monthly mean values of temperature, photoperiod, light intensity, relative humidity and rainfall were noted, from which the summer season mean values were calculated for the time period of the experimental period (Table 1).
|
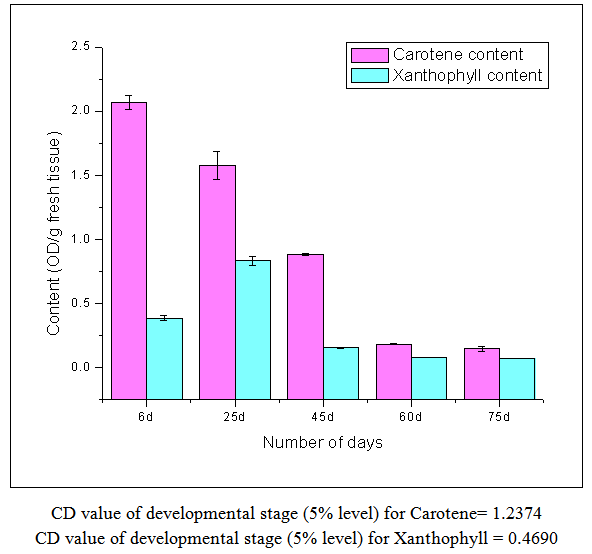 | Figure 1. Carotene & Xanthophyll content |
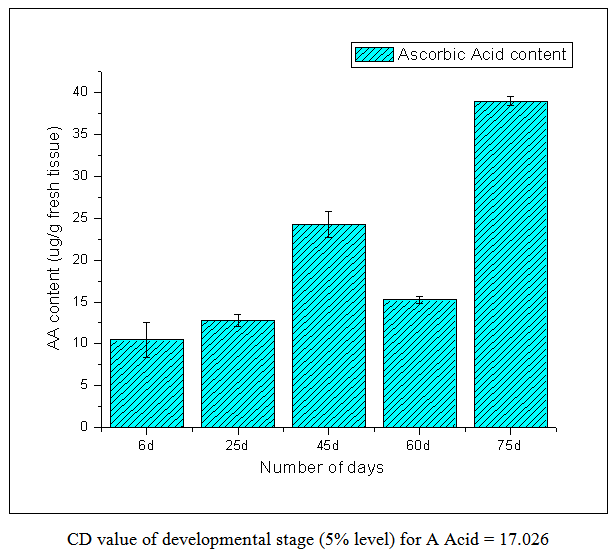 | Figure 2. Ascorbic Acid Content |
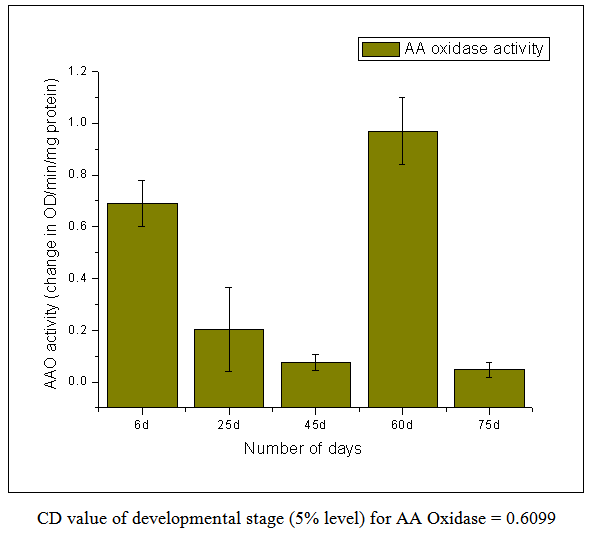 | Figure 3. Ascorbic Acid Oxidase Activity |
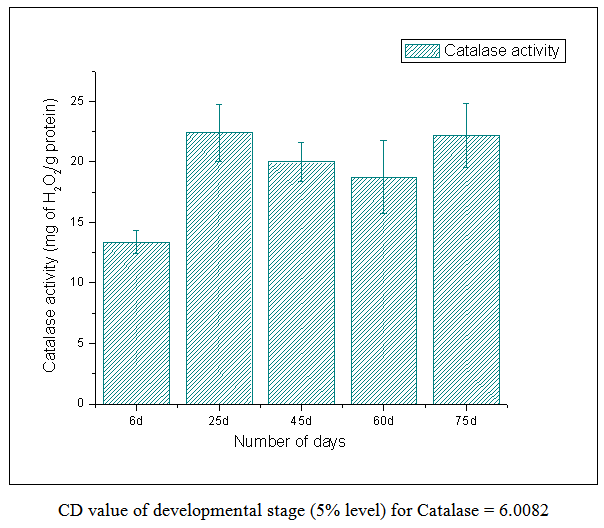 | Figure 4. Catalase Activity |
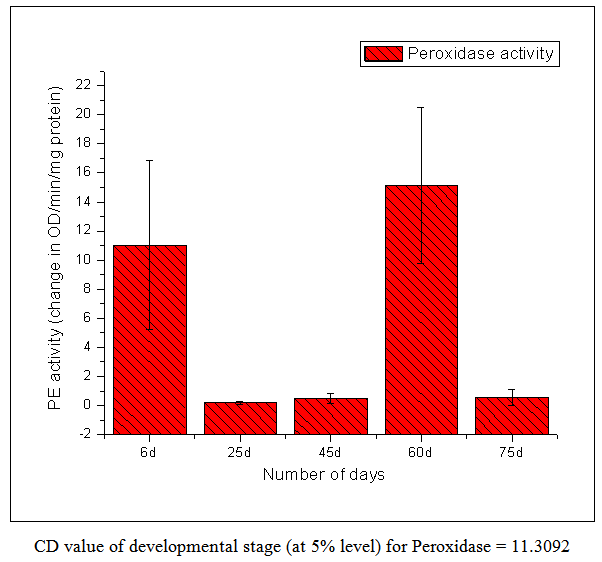 | Figure 5. Peroxidase Activity |
4. Conclusions
- From this work, it indicates 60 DAS to be the point in the plant life cycle when the plant shifts from an optimum phase (active growth) to a stressful condition (comparable to post flowering/senescence). This is amply justified by the low ascorbic acid content accompanied by low carotenoid content i.e. both kinds of non-enzymatic antioxidants are significantly low as compared to the previous favorable stage of 45 DAS. Both the scavenging enzymes viz. catalase and peroxidase show elevated activities at this particular stage i.e. 60 DAS in order to detoxify the free radicals and combat the existing natural stress of senescence. Ascorbic acid oxidase activity is also high which is the cause of low ascorbic acid content. From this work, it is also evident that the period from 25-45 DAS is the most favorable in terms of antioxidant profile and scavenging enzymes. Thus the physiological status is optimally maintained during this phase resulting in active growth and flowering. As our work indicates the favorable period (25-45 DAS) as well as the stressful (60 DAS) stage (post flowering leading to senescence) in the Vigna radiata life cycle in terms of antioxidant profile and scavenging enzymes, these findings may contribute in the future research about stress physiology and senescence of this particular legume.
ACKNOWLEDGEMENTS
- We thankfully acknowledge Prof. Buddhadeb Chattopadhyay, Principal, Govt. College of Engineering & Leather Technology (GCELT), for his valuable guidance and assistance. We are thankful to GCELT for using Biotechnology Lab and Project Lab. We acknowledge Prof. S. S. Das, School of Mathematics, Uluberia College, for his assistance in statistical analysis. Meteorological data from Regional Meteorological Centre, Alipore, Kolkata is also gratefully acknowledged. We wish to thank UGC for their financial support (UGC Minor Project: PSW 49/ 12-13 ERO: dated 5th Feb, 2013).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML