-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2014; 4(2): 51-55
doi:10.5923/j.plant.20140402.02
Effects of Soil Type and Different Pre-sowing Treatments on Seedling Emergence and Vigour of Acacia sieberana
Innocent Pahla1, Tavagwisa Muziri1, Tiniel Chinyise1, Simbarashe Muzemu1, James Chitamba2
1Department of Horticulture, Faculty of Natural Resources Management and Agriculture, Midlands State University, P. Bag 9055, Gweru, Zimbabwe
2Department of Agronomy, Faculty of Natural Resources Management and Agriculture, Midlands State University, P. Bag 9055, Gweru, Zimbabwe
Correspondence to: Innocent Pahla, Department of Horticulture, Faculty of Natural Resources Management and Agriculture, Midlands State University, P. Bag 9055, Gweru, Zimbabwe.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
An experiment was carried out at Midlands State University to determine the effect of soil type and different seed treatments on seed emergence and seedling vigour of Acacia sieberana. The experiment was arranged as a 2×5 factorial treatment structure laid out in a completely randomized design with three replications. Soil type had two levels (sand and clay) while seed treatments had five levels (98% H2SO4 for 60 minutes, 98% H2SO4 for 90 minutes, hot water only, hot and cold water, and straight sowing (control)). Results showed that soil type and seed treatment significantly affected seed emergence (p<0.05) of A. sieberana. Seeds subjected to 98% H2SO4 for 60 minutes and then sown in sand soil gave the highest emergence percentage of 80%, followed by those that were hot water treated and sown in sand soil with 53%. Soil type and seed treatment significantly affected days to emergence (p<0.05) of the A. sieberana. Seeds treated with 98% H2SO4 for 60 minutes and then sown in sand soil had the shortest period to emergence (6 days). Straight sown seeds recorded the longest time to emergence (18 days). It was concluded that subjecting seeds to 98% H2SO4 for 60 minutes and sowing them in sandy soils can improve emergence percentage and also reduce time taken to emergence of A. sieberanaseeds.
Keywords: Acacia sieberana, Soil Type, Seed Pre-treatment, Germination, Emergence
Cite this paper: Innocent Pahla, Tavagwisa Muziri, Tiniel Chinyise, Simbarashe Muzemu, James Chitamba, Effects of Soil Type and Different Pre-sowing Treatments on Seedling Emergence and Vigour of Acacia sieberana, International Journal of Plant Research, Vol. 4 No. 2, 2014, pp. 51-55. doi: 10.5923/j.plant.20140402.02.
Article Outline
1. Introduction
- Acacia sieberana is an important tree species for social forestry and agroforestry program due to its rapid growth and capability of nitrogen fixation. The genus Acacia has more than 500 species found throughout the world, with most being native to Australia. However, some of the most attractive of these trees and shrubs are native both to Zimbabwe and South Africa [1]. There are several species within this genus of acacia and these include Acacia albida; A. galpinii; A. polyacantha; Acacia karoo; Acacia nitotica; Acacia tortilis and A. sieberana. A. sieberana is flat topped and can grow up to 9 m high with a spread of approximately 15 m. A. sieberana pods and leaves can be used to feed livestock and game; stem and branches as bush fencing. The tree also has some aesthetic and practical value as shade around homesteads as well as the bark having some medicinal properties [1]. The fact that A. sieberana is deciduous means that it can bring different forms and impacts in the garden during the different seasons in the year. Van Vyk et al. [2] noted that a leafless tree is best able to express its form. The tree casts a dappled shade which permits a lawn to grow beneath the canopy and also tolerates drought. Moreover, the tree has some soil improving properties and can live up to 100 years, can coppice easily, thus making it a suitable landscape plant for degraded environments [3].However, all species of acacia are propagated from a seed with an extremely hard seed coat, which under natural conditions would take a long time to germinate [4]. The factors which affect the germination of Acacia species in a given micro-environment are soil type, seed dormancy and water availability [5, 6]. Substantial reduction in seed germination has been shown in many species [7]. The situation is exacerbated by the fact that seeds are the only means of propagation of Acacia species [8] hence seed dormancy could present a serious problem for seed germination of A. sieberana thereby affecting establishment of this agroforestry tree species.Germination of A. sieberana is very poor as the seeds are dormant hence they will not germinate promptly when subjected to conditions which are normally regarded as suitable for germination. Therefore, the seeds must be artificially subjected to some physical and chemical pre-sowing treatment so as to cope up with dormancy and obtain uniform, rapid and synchronous germination [9, 10]. External stimuli to promote seed coat rapture of hard seeded species like A. sieberana is thus required [11].The fact that A. sieberana is propagated from a hard coated seed reduces the scope of its use as a landscape plant. This is in spite of the fact that the tree is capable of providing obvious aesthetic and ornamental values in the landscape environment. However, the most limiting factor to its use in landscapes is its lack of readiness to germinate. Yet, problems of low germination rates for several tropical agroforestry tree species are still caused by dormancy, partly because of lack of general knowledge of their seed physiology, and partly because of variation in dormancy rate [12]. The effect of different pre-treatment methods on seed germination of some tree species has been reported [10, 13, 14]. However, there are few publications on the effect of different soil types and pre-sowing treatment of A. sieberana hence intensive plantation of the crop in Agroforestry and home garden is restricted due to poor seed germination and delayed initial establishment in the nursery [13, 15]. Therefore, the main objective of the present study was to determine the best possible soil type and pre-sowing treatment method that maximizes the seed germination and initial seedling growth of A. sieberana in the nursery.
2. Materials and Methods
- The experiment was carried at the Midlands State University, which is located in the Midlands Province in Zimbabwe, the area is in agro-ecological region III. It is at an altitude of 1428 m, latitude of 19ºE and longitude of 29º85′. The area receives an average annual rainfall of between 550 and 600 mm. Its mean temperature is 22ºC. The experiment was carried out as a 2×5 factorial experiment laid in a completely randomized design (CRD). The experiment had two factors, soil type with two levels (clay and sand) and seed treatment with five levels (98% H2SO4 for 60 minutes (HSC1), 98% H2SO4 for 90 minutes (HSC2), hot water only (HT), hot and cold water (HC), and straight sowing (SS) (control)).Thirty seeds were placed in 98% H2SO4 for 60 minutes and another Thirty for 90 minutes and then washed repeatedly and thoroughly in cold water. For the hot water treatment, thirty seeds were put in hot water, left in the water till the water cooled down. For the hot and cold water treatment, thirty seeds were placed briefly (2 minutes) in hot water, and then transferred immediately into cold water. After being subjected to treatments the seeds (including the direct sowing were further soaked in cold water for 30 minutes, after that the water was drained and seeds were allowed to dry for 60 minutes.Black polythene bags that were 10 cm wide by 25 cm long were filled with equal amounts of growing medium, that is, sand and red clay. Fifteen polythene bags were used for each soil type; equal amount of soil was applied in the bags after which planting holes of 2 cm deep were made. Ten seeds (subjected to treatments) were sown in each polythene bag and covered with soil. Watering was done to field capacity to initiate germination and this was done on the same day for all treatments.The number of days taken to 30 % germination was noted. The germination percentage was determined after 14 days from planting day. It was determined using the following formula:
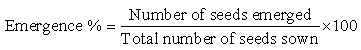 Height of seedlings was measured at 21 days after emergence of the first seedling.The data was subjected to analysis of variance using GenStat statistical package version 7.22 and the least significant difference (LSD) was used for separation of treatment means at 5% significance level.
Height of seedlings was measured at 21 days after emergence of the first seedling.The data was subjected to analysis of variance using GenStat statistical package version 7.22 and the least significant difference (LSD) was used for separation of treatment means at 5% significance level.3. Results
- There was a significant interaction (p<0.05) between soil type and seed treatment on the number of days to 30% germination. Seeds that had been exposed to H2SO4 for 60 minutes and planted in sand soil took 6 days to emerge, whilst seeds exposed to hot and cold water treatment and planted in clay soil took the longest time to emerge (18 days) (Figure 1). Seeds that were treated with hot/cold water, hot water, 98% H2SO4 for 60 minutes and 98% H2SO4 for 90 minutes and planted in sand soil recorded lower number of days to emergence compared to the seeds exposed to the same treatments but planted in a clay soil (Figure 1).
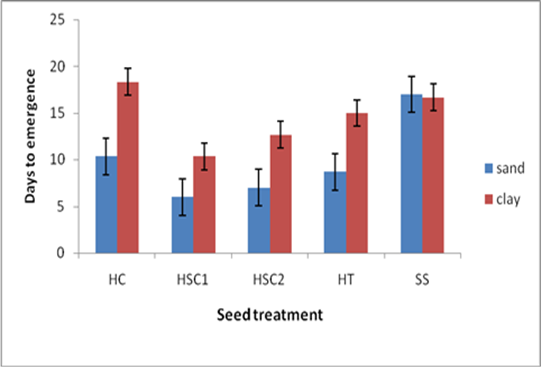 | Figure 1. Effect of soil type and pre-sowing seed treatment on days to 30% seedling emergence |
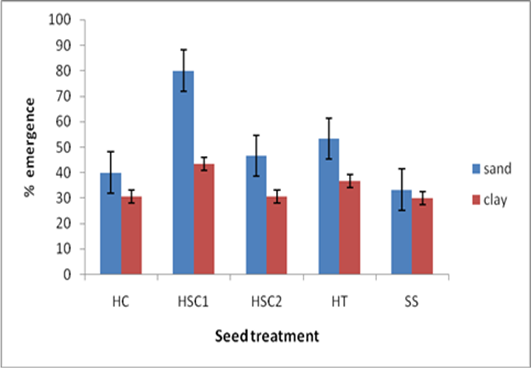 | Figure 2. Effect of soil type and pre-sowing seed treatment on emergence percentage |
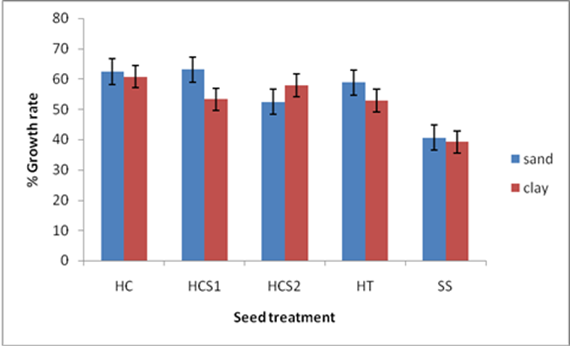 | Figure 3. Effect of soil type and seed treatment on seedling shoot height |
4. Discussion
- Soil type had a significant effect on days to emergence with the sand soil planted seeds taking the shortest time to emerge across all seed treatments as compared to clay planted seeds. This could be because of the physical and chemical characteristics of clay. Dey [16] highlighted that clay can pause a major challenge to germination and emergence due to its poor drainage, and it tends to clod easily. An ideal propagation media must be sufficiently porous so that excess water drains away, permitting adequate penetration of oxygen to the seed, of which all these attributes are lacking in clay soils. All containers used as propagation potting plants produce a perched water table that creates a zone of saturated growing medium at the bottom of the container, more so, if the soil has poor drainage like clay [17].Seeds treated with H2SO4 took the shortest time to emergence in both soils. However the best results were recorded where seeds were subjected to 98% H2SO4 for 60 minutes and then sown in sand soil. Baskin [18] noted that the whole idea behind treating the seeds is to either completely remove the germination impeding seed coat or to reduce its thickness so that the seed could emerge. Removal or reduction in thickness of the seed coat allows the seed to take up water and respiratory gases thus the germination process can be initiated. It is highly probable that treating seeds with H2SO4 reduced the thickness of the seed coat compared to the other scarification techniques. Exposing the seeds to concentrated levels of sulphuric acid can also have a negative effect as it can end up damaging the seed, when the acid can penetrates into the seed via its exposed micropyle [19]. This could possibly explain why seeds that were exposed to 98% H2SO4 for a longer period of 90 minutes recorded a lower emergence rate as compared to those exposed for 60 minutes in the same concentration.Seeds sown in sand soil performed better than those sown in clay soils across all treatments. The results are similar to Okello and Young [20] who observed that A. drepanolobium seed germination was higher in red sandy soils than in clay loams. Sand has loosely aggregated particles that allow free exchange of gases between the germination medium and the embryo. Oxygen is essential for respiratory purposes in germinating seeds and oxygen uptake is proportional to the amount of metabolic activity taking place [17]. Sand soil is the most suitable germination medium for tropical tree seed germination due to its availability, low cost, capacity to hold moisture and suitability for large tree seeds [21].Poorly drained soils like clay have their pore spaces filled with water so that little oxygen is available to seeds, where the amount of oxygen in the medium is affected by its low solubility in water and its ability to diffuse in the medium [22]. Landis et al. [23] noted that gaseous exchange between the germination medium and the atmosphere, where oxygen concentration is 20%, is reduced significantly by hard crust on the surface which can limit oxygen diffusion. This coupled with treating seeds with sulphuric acid for one hour could have aided an increase in germination percentage in seeds treated with H2SO4 for 1 hour and then sown in sand soil, since this treatment recorded the highest percentage germination of 80%. Acid can effectively reduce the seed coat thickness, allowing the seed to take in oxygen and water through the micropyle [24]. This is consistent with Niranjan et al. [9] who reported that pregermination treatments like H2SO4 will destroy the integrity of impermeable cover and so permit the imbibition of the embryo. On the other hand, because clay particles are closely packed together and remain saturated with moisture for long periods of time, they are therefore slow to warm up, thus temperatures for germination are not attainable easily.Hot water treatment also showed a significantly high germination percentage as compared to control because it simulates the effect of veld fires on the seed where the seed coat is burnt and reduced in thickness, allowing the seed to take in oxygen and water, thus breaking its dormancy [25]. Leaving the seed soaked in water has the possibility of leaching out metabolites that inhibit germination [26]. However, the findings are inconsistent with Azad et al. [13] who reported highest germination (52%) in hot water treatment in A. lebbeck. This may be due to the variation of seed coat thickness of the species used in the latter and the present study. Moreover, Schmidt [12] reported that hot water overcomes physical dormancy in leguminous plants like A. sieberana by creating tension which consequently causes cracking of the macrosclerid layer or by affecting the strophiolar plug.Soil type and seed treatment had no significant effect on the subsequent seedling height in the twenty one days of observation from planting. This could be because during this period, all the seedlings that had germinated were still drawing their nutrients from the seed endosperm, thus eliminating the effect of soil on their growth rate [26]. Moreover, all the seed treatments did not have any effect on the endosperm, thus giving a shoot height that is not significantly different from each other.
5. Conclusions
- Basing on these research findings, it could be concluded that treating seeds with H2SO4 for an hour and then planting them in sand soil is the most ideal pre-sowing treatment and propagation media respectively for A. sieberana seed. This treatment the least number of days to emergence and gave comparatively highest germination percentages. Treating seed in hot water and then planting in sand soil proved to be a competent and superb alternative propagation combination. It could be recommended that for the propagation of A. sieberana by seed, sand soil combined with H2SO4 pre-sowing treatment for one hour can be used.
ACKNOWLEDGEMENTS
- The authors would like to acknowledge the management and staff of Midlands State University for provision of expertise and necessary materials/resources for the experiment to be successfully carried out.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML