-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2014; 4(1): 5-10
doi:10.5923/j.plant.20140401.02
Natural Products – New Antimicrobial Constituents Derived from Cerium arvense
Zia Ul Haq Khan1, Shafiullah Khan2, Yongmei Chen1, Sadaf Ul Hassan2, Pingyu Wan1
1School of Science, Beijing University of Chemical Technology, Beijing, 100029, China
2State key Laboratory of Organic-Inorganic Composites, Beijing University of Chemical Technology, Beijing, 100029, China
Correspondence to: Pingyu Wan, School of Science, Beijing University of Chemical Technology, Beijing, 100029, China.
| Email: | 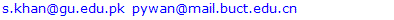 |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abtract A new spectrum of human bacterial and fungal infections is increasing due to the increase of different ailment; AIDS, cancer, and neurodegenerative disease like Alzheimer,s patients. The increase use of antibacterial and antifungal agents also resulted in the development of resistance to the present drugs. It make necessary to discover new classes of antibacterial and antifungal compounds to cure the fungal infection. Therefore, research on natural products and isolated a series of two new bioactive compounds Arvense A (1) and Arvense B (2) were isolated from n-butanol soluble fraction of Cirsium arvense along with two know compounds 5,4'-Apigenin 7-O-β-D-glucoside (3), Dihydroxy-6,7-dimethyoxyflavone 4'-glucoside (4). Their structures have been identified by electrochemical impedance spectroscopy (EIS), high resolution resonance ionization mass spectrometry (HR-EIMS), 1H-NMR and 13C-NMR spectroscopic methods. The antibacterial and antifungal activities of these compounds were tested; this compound shows profound antimicrobial activities.
Keywords: Cirsium arvense, Butanol soluble fraction, Arvense A and B, Antibacterial activity antifungal activity
Cite this paper: Zia Ul Haq Khan, Shafiullah Khan, Yongmei Chen, Sadaf Ul Hassan, Pingyu Wan, Natural Products – New Antimicrobial Constituents Derived from Cerium arvense, International Journal of Plant Research, Vol. 4 No. 1, 2014, pp. 5-10. doi: 10.5923/j.plant.20140401.02.
Article Outline
1. Introduction
- Cirsium arvense is a medicinal plant of family Asteraceae [1] and is often found as noxious weed in grasslands and riparian habitats[2]. C. arvense is known to reduce forage biomass[3] as well as its favorable response to fertilization[4, 5]. A recent study shows that the foliar endophytic fungal community composition in C. arvense is affected by mycorrhizal immigration and soil nutrient content[6]. The genus Cirsium L. have various medicinal uses especially for the treatment of peptic ulcer and leukaemia in folk medicine [7] epitasis, metrorrhagia, syphilis eye infections[8] skin sores gonorrhoea, bleeding piles and has also been found to be effective against diabetes[9,10]. Americans and Indians allegedly used an infusion of C.arvense roots for mouth diseases, worms and poison-ivy (Toxicodendron radicans) and in treatment of tuberculosis[11]. C. arvense roots are reported to having arsenic resistant bacteria[12]. A recent study discovered that nonenolides and cytochalasins exhibited strong phytotoxic activity against C.arvense leaves [13]. Scent of C. arvense attracts both floral herbivores and pollinators[14]. C.arvense is found in very considerable quantities in district Bannu Pakistan. Its local name is “Aghzikai”. Prior studies recommended that flavonoid compounds, phenolic acids, tannins, sterols and triterpenes are the main constituents of genus Cirsium[15,16,17]. The various therapeutic uses attributed to this species prompt us to carry out phytochemical exploration and biological activities of constituents of this plant. The methanolic extract of the whole plant of C. arvense showed significant toxicity in brine shrimp lethality test[18].In our previous work, several antibacterial and antifungal compounds have been isolated from the chloroform fraction of the methanolic extract of the plant[19]. We also reported our further work on EtOAc fraction[20]. After being separated by column chromatographic method, four compounds were isolated from n-butanol soluble fraction and their antibacterial and antifungal activities were tested. These compounds are first time isolated from the plant.
2. Materials and Methods
2.1. General Experimental Procedure
- The precoated silica gel F254 plates were used for TLC, Silica gel (E-Merck, 230-400 mesh) was used for column chromatography. Melting points were determined on a Gallen kemp apparatus and are corrected. The UV spectra (λmax nm) were recorded on Hitachi UV-3200 spectrophotometer in MeOH. The IR spectra (ν max cm-1) were recorded on Jasco-320A spectrophotometer in CHCl3. The mass spectra were recorded on a Varian MAT 312 double focusing mass spectrometer connected to DEC-PDP11/34 computer systems. The 1H-NMR and 13C-NMR spectra were recorded on a Bruker AM-300 HNMR spectrometer (300 MHz for 1H and 75MHz for 13CNMR) using CDCl3 as solvent. The assignments were made by distortion less enhancement by polarization transfer (DEPT), correlation spectroscopy (COSY) and hetero nuclear multiple bond correlation (HMBC) experiments. Optical rotations were measured on Jasco-DIP-360 digital polarimeter using a 10 cm tube. Ceric sulphate and aniline phthalate were used as detecting reagents.
2.2. Plant Materials
- The plant material was collected from Musa Khel Bannu (Pakistan) and identified by Muhammad Yousf Khan Professor in Botany Government Post Graduate College Bannu. The specimen (NO: 230) was deposited in the Herbarium of Botany Department in Government Post Graduate College Bannu.
2.3. Extraction and Isolation
- The shade dried plant of C. arvense (8kg) was ground and extracted with MeOH (32 L×3) at room temperature. The combined methanolic extract was evaporated under reduced pressure to obtain a dark brown gummy material (650g). The gummy material was suspended in water and extracted with n-hexane (115g), CHCl3 (98g), EtOAc (82g) and n-butanol (60g) soluble fractions, respectively. The fractions were then placed in a vacuum oven at not more than 40°C for about 24h to remove any residual solvent. These fractions were screened for toxicity. The CHCl3 and EtOAc soluble fractions show highly toxicity[19]. The ethyl acetate (EtOAc) soluble fraction was subjected to column chromatography over silica gel (70-230mesh) eluting with n-hexane, n-hexane: EtOAc, EtOAc: CHCl3 and EtOAc, EtOAc: MeOH and MeOH in increasing order of polarity to obtained sub-fractions (1 to 4).The n-butanol soluble fraction was subjected to column chromatography over silica gel (70-230 mesh) eluting with n-hexane, Chloroform: Butanol, and Butanol: ethyl acetate, butanol, butanol: methanol increasing order of polarity to obtained sub-fraction (A-D). The sub-fraction B, (100% butanol 12g) was again chromatographed over silica gel with n-hexane: butanol, butanol: Chloroform, butanol ethyl acetate and butanol, in increasing order of polarity to obtain A'-D'. The fraction A' (3g) obtained from butanol: chloroform (5.5:3.5) was again subjected to column chromatography over silica gel eluting with mixture of n-hexane: butanol: chloroform and butanol, in increasing order of polarity. The fractions which eluted with butanol: chloroform (6.5:3.5) were combined and loaded on preparative TLC in solvent system n-hexane: Acetone: Ethanol (3.5:3.5:3) yielded, arvense A (1) (10mg). The fraction B' obtained from butanol: ethyl acetate was again subjected to column chromatography over silica gel eluting with mixtures of n-hexane: butanol: chloroform and butanol in increasing order of polarity. The fractions obtained from butanol: chloroform (5.5:4.5) showed a major spot on TLC using n-hexane: ethanol: diethylamine (4.7:5:3) as solvent system, afforded arvense B (2) (8mg). The sub-fraction C' (3g) obtained from butanol (100%) was re-chromatographed over silica gel eluting with mixture of n-hexane: butanol, butanol: chloroform and butanol: acetate in increasing order of polarity. The fractions obtained from butanol: ethyle acetate (4.7:5.3) were mixed and showed a major spot on TLC. It was concentrated and subjected to preparative TLC using n-hexane: butanol (4.3:5.7) as solvent system to afford 3 (5mg). The sub-fraction D' n-hexane: butanol (3.6:6.4) was re-chromatographed over silica gel eluting with n-hexane: butanol, butanol: chloroform and butanol in increasing order of polarity. The fractions obtained from butanol:CHCl3 (5.4:4.6) were mixed and concentrated. The fraction obtain from butanol: chloroform (4.5:5.5) were subjected to preparative TLC using n-hexane: acetone: methanol (2.5:1.8:5.7) as solvent system to afford compound 4 (9 mg).
2.4. Anti-bacterial Assay
- ProcedureThe antibacterial activities were determined using ager well diffusion (AWD) method[21]. The procedure consists of the following steps. First bacterial culture was grown in nutrient broth at 37°C for 18-24 hours. 2. 0.5 ml of broth culture of test organism was added by sterile pipette into molten agar of 50 ml, which are than cooled to 40°C and poured in to sterile Petri dish. Sterile cork borer was used to make well of 6 mm in diameter in each nutrient ager plate. The wells were filled with given compounds (100 µl) and the plates were allowed to stand for 1-2 hours. At last, the plates were incubated at 37°C for 18-24 hours. Finally the diameter of inhibition was measured.
2.5. Anti-fungal Assay
- ProcedureSterile DMSO was used to dissolve the test sample. SDA (Sabouraud dextrose agar) was prepared by mixing Sabouraud 3% glucose agar and agar-agar in distilled water [22]. The required amount of fungal strain was suspended in 2ml sabaouraud dextrose broth. This suspension was uniformly streaked on Petri plates containing sabaouraud dextrose agar media by means of sterile cotton swab. Compounds were applied in to well using same technique for bacteria. These plates were than seen for the presence of zone of inhibitor and results were noted.
3. Result and Discussion
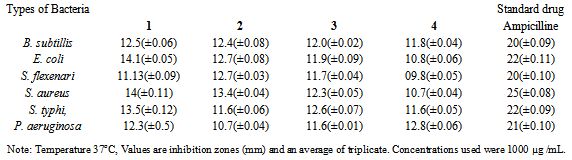
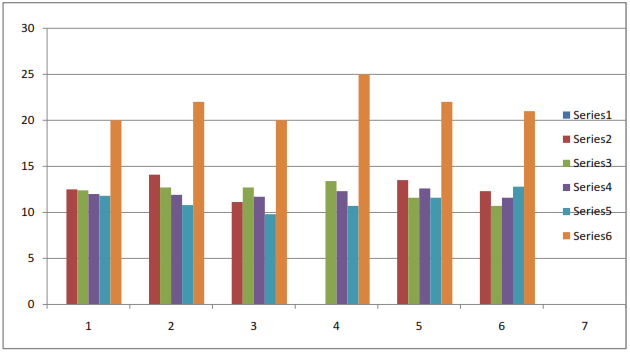
- The n-hexane, CHCl3, EtOAc and n-BuOH soluble fractions were taken of methanolic extract. The repeated column chromatography and preparative TLC using silica gel on n-BuOH soluble fractions resulted in the isolation and characterization of two new bioactive compounds arvense A (1) and arvense B (2) along with two know compounds 5,4'-Apigenin 7-O-β-D-glucosid (3), Dihydroxy-6, 7-dimethyoxyflavone 4'-glucoside (4). The IR spectrum of 1 showed bands 1078cm-1 corresponding to hydroxyl group and 1616 cm-1of double bonds. From EI-MS, 1H-NMR and 13-NMR spectra data, the molecular formula was deduced to be C29H50O2. The IR spectrum of 2 showed bands corresponding to 1730cm-1 ᵧ-lactones ring and double bonds 1615 cm-1. From EI-MS, 1H-NMR, 13C-NMR and 2D spectral data, the molecular formula was deduced to be C14H18O3. The signal of 3.85(1H, t, J =9.5 H-6) and 2.60(1H, dt, J =12.0, 5.1Hz) was indicative of a lactone ring. These four compounds were first time isolated from this plant. The antibacterial activity of these compounds was performed against Bacillus subtillis, Escherichia coli, Shigella flexenari, Staphylococcus aureus, Salmonella typhi, and Pseudomonas aeruginosa. As shown in Table 1. The zone of inhibition of these compounds were measured compared with standard drudge. The zone of inhibition of 1 and 2 is almost same and 3 showed good activity, while 4 showed moderate activity. The antifungal activity of compounds (1 to 4) was performed against six pathogenic fungi, Trichophytonlongifusus, Candida albicans, Aspergillus flavus, Microsporum canis, Candida glabrata and Fusariumsolani Table 2. The antifungal result showed same result as antibacterial.
 | Figure 1. Structure of compounds 1-4 |
|
|
 | Figure 2. Important HMBC correlation in compound 1 and 2 (H → C) |
 | Figure 2. Antibacterial activity of compounds 1-4 isolated from cirsium arvense |
 | Figure 3. Antifungal activity of compounds 1-4 isolated from cirsium arvense |
4. Conclusions
- Several antimicrobial active compounds were isolated from cirsium arvense, which shows profound activity. The chloroform and ethyl acetate soluble fractions contain active compounds. In the present work we isolated several compounds from n-butanol soluble fraction which shows good antimicrobial activity. We recommended further investigation on this medicinal plant.
AKNOWLEDMENTS
- The authors thank the Chinese Scholarship Council (CSC N0:2012GXZ174) for financial support of this research work.
Conflict of Interest
- The authors report no conflict of interest.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML