-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2014; 4(1): 1-4
doi:10.5923/j.plant.20140401.01
Introgression of bsr Gene from Wild 2n Accessions and Derivative Hybrids to Cultivated 3n Landraces of Plantains (Musa Sp.)
Orluchukwu J. A.1, Ogburia M. N.2
1Department of Crop and Soil Science, Faculty of Agriculture, University of Port Harcourt, P. M. B. 5323, Port Harcourt, Nigeria
2Department of Crop and Soil Science, Rivers State University of Science and Technology, P.M.B 5080, Port Harcourt, Nigeria
Correspondence to: Orluchukwu J. A., Department of Crop and Soil Science, Faculty of Agriculture, University of Port Harcourt, P. M. B. 5323, Port Harcourt, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Plantains and bananas are important crop for global trade and nutrition where they are intensively cultivated but little effort exist to breed superior plantains and banana. The main objective of the present research which was investigated at the Teaching and research farm of the Rivers State University of Science and Technology, Port Harcourt, Nigeria was to produce hybrids that are resistant to black sigatoka disease (BSD) through the introgression of black sigatoka resistant (bsr) gene from wild diploid accessions and derivative hybrids to cultivated triploid landraces of plantain. All plantain cultivars and most triploid bananas are susceptible to black leaf streak disease. Plantain and banana improvement programs make use of interspecific hybridization for gene introgression. Consequently, the triploid plantain landrace, Agbagba, Valery, Unknown female plantain, Bluggoe, Calcutta 4, French, km5 (Yangambi) and USTPx 02/01 were crossed with a wild diploid banana, Calcutta 4, km5 (Yangambi), Unknown male plantain to generate good disease resistant hybrids. Out of 152 seeds produced from the crosses only four (%) germinated, and were established in the field. The established hybrids were assessed for black sigatoka disease reaction and it was found out that one (1) hybrid was resistant while three (3) were partially resistant. Therefore, introgression of bsr gene is feasible.
Keywords: Plantain, Introgression, Black sigatoka resistant gene, Hybrids
Cite this paper: Orluchukwu J. A., Ogburia M. N., Introgression of bsr Gene from Wild 2n Accessions and Derivative Hybrids to Cultivated 3n Landraces of Plantains (Musa Sp.), International Journal of Plant Research, Vol. 4 No. 1, 2014, pp. 1-4. doi: 10.5923/j.plant.20140401.01.
1. Introduction
- Plantains and bananas (Musa spp.) are perennials which produce generations of ratoo crops. They represent the world’s second largest fruit crop with an annual production of 129,906,098 metric tons[4]. They rank as the fourth most important global food commodity after rice, wheat and maize in terms of gross value of production[8].The Plantain and bananas are affected by a number of diseases and pests primarily black sigatoka caused by Mycosphaerella fijiensis, fusarium wilt, nematodes, weevils and viruses. Farmers’ opportunity to increase their incomes is impeded by the high pest and disease susceptibility of the local plantain cultivars. Currently black leaf streak (Mycosphaerella fijiensis) and the burrowing root nematode (Radopholus similis) are considered major causes of yield losses in plantain production. In commercial sweet banana production, both problems are controlled with pesticides. This, however, appears unprofitable in smallholder settings [7]. Thus, genetic improvement through introduction of multiple pest and diseases resistant or tolerant cultivars may offer suitable options.Cross breeding of bananas is essential to produce improved hybrids that would be resistant to the various pests and diseases. Crossing a triploid banana with diploid accessions generates diploid, triploid, tetraploid, aneuploid and hyperploid progeny[25].Many pests and diseases have significantly affected Musa cultivation. Black sigatoka, a leaf spot disease caused by the fungus Mycosphaerella fijiensis (Morelet), is generally considered to be the most serious constraint to plantain and banana production in sub-Sahara Africa. First identified in Fiji, the disease was accidentally introduced into Southern Africa in the 1 970’s and spread rapidly first in Central and West Africa, and later in East Africa[10]. Once established, the pathogen causes severe leaf necrosis, reducing yields by 30-50%. Black sigatoka disease has become a major constraint to banana and plantain production worldwide resulting in yield losses of about 33 to 50%[10; 17]. All plantain cultivars and some of the most popular banana cultivars of East Africa are susceptible to black sigatoka[21; 26]. As a consequence of these threats to Musa cultivation, the aim of this experiment was to produce plantain Musa hybrid that are resistant to black sigatoka disease, and more so to determine the resistance level of the hybrid to black sigatoka disease.
2. Materials and Methods
- The experiment was conducted in the Teaching and Research Farm of the Rivers State University of Science and Technology, Port Harcourt. Rivers State has a landmass of 19420 sq.km[11], and lies within tropical rainforest zone of Nigeria, located in latitude 4°-6°N and longitude 6°-8°E. The project site (University Farm) is located on latitude 4.5°N and longitude 7.01°E with an elevation of 1.8m above sea level[5].The rainfall pattern is essentially bimodal with peaks in June and September, while in April and August there are periods of lower precipitation[23] and[24]. The long rainy season is between April and October. The dry season lasts from November to March with occasional interruption by sporadic down pours. Annual rainfall is average of 2000mm to 4500mm[1]. The mean monthly temperature ranges between 28℃ and 33℃ while the annual monthly minimum is between 20℃ and 23℃. The highest temperatures are experienced during the months of December through March and coincide with the overhead passage of sun[3]. A total land area measuring 42m x 50m (2100m2) was cleared and used for the experiment. The land area was cleared manually, marked and pegged. The experimental materials are made up of eight[8] different Musa cultivars. The data were arranged in a Complete Randomized Block Design and analysed using appropriate computer software.The initial soil samples were collected and analysed for Soil pH, Total Nitrogen, Potassium and Available Phosphorus. The aim was to determine the initial nutrient status of the soil. Different clones of suckers were collected from the Research and Teaching Farm at the Rivers State University of Science and Technology, Port Harcourt. The clone varieties or cultivars used for the research were Valery, USTPx 02/01, Calcutta 4, Nkpolu Banana, Bluggeou, Km5 (Yangambi) and Plantain landrace. These parental clones were selected due to their relatively high female fertility[20, 22]. However, diploid (2n=2x=22) male parent Km5 (AA) (xx) were used as a source of black sigatoka resistant (BSR) due to its high level of resistance to the disease[6; 15; 20]. Furthermore, Km5 possesses two complementary dominant genes for parthenocarpy[16) but is not expressed in edible fruit production. Again, it produces ample pollen that is 100% viable.The prepared corms were left to dry for two[2] days (not in the sun) before planting. Average weight of suckers was 3-4kg and is predominantly late sword suckers according to Obiefuna and Ndubizu[12]. The late sword suckers are known to be the best planting material for plantain.Planting was done in May 2009 at a spacing of 3m x 2m. Planting holes were prepared with a minimum size of 30cm x 30cm x 30cm[19]. The crop was planted in rows. A total of fourteen[14] rows were planted with twenty-five[25] stands per rows. This gave a plant population of 350 stands per 2100m2. At maturity of the plant, i.e. +90 days after shooting[13], pollen grains of fleshly exposed male flowers were collected on daily basis within the morning hours of 7- 10 in the morning using ladder to reach the tall plants.Different crosses were carried out. At the end of the period, seeds were produced in some of the pollinated plants. The seeds produced were planted in a plastic container with perforation at the base. The container was measuring 20cm by 20cm by 20cm.The plastic containers were filled with top soil rich in organic manure up to 2/3 height. Before planting, the seeds were soaked in water, and some of the seeds floated. About 3 seeds planted per container.After one month of planting, seed germination was observed in the cultivars. Though, some of the seeds failed to germinate. The germinated seeds were transplanted to the field after two months. Holes were prepared about 30cm x 30cm x 30cm and filed with top soil. The seedlings were planted by “ball of earth” method as the plastic containers were carefully removed, and seedling placed firmly inside the holes. Planting space adopted was 2m x 3m.
3. Results and Discussion
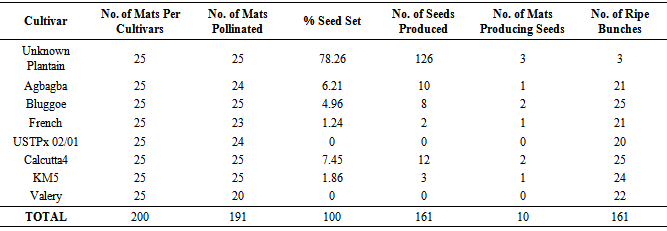
- Seed productionThe cultivars crossed are Valery, Calcutta 4, Yangambi (km5), USTPx 02/01 and unknown plantain. During harvest, it was discovered that not all the stands pollinated were able to produce seeds. Nevertheless, seed set percentage were calculated based on each cultivar. Formula for the calculation is:
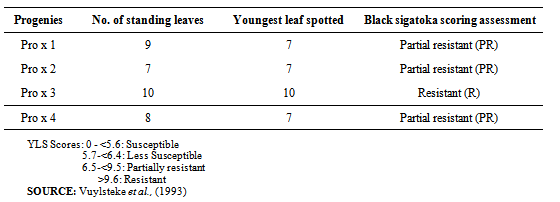
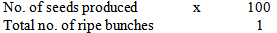 In Table 1, it is evidenced that unknown plantain produced the highest seed of 126 followed by Calcutta 4. Others followed a decreasing order of seed production respectively. Seed setting in Musa genotypes is always difficult. This is partly due to chromosomal imbalance since most of the cultivated Musa species are triploids especially the plantains [18].Based on Table 1, Calcutta 4 and unknown plantain produced many seeds while Agbagba, Yangambi (km5), Bluggoe and French plantain produced very few seeds. Even some of the few seeds were found floating in water meaning that they were not viable. This is in line with Dessauw[2] report, that female fertility in French plantains is very low and absolute in horn type. In contrary to this, Swennen and Vuylsteke[20] working at IITA have identified 12 females fertile French plantains although they concluded that fertility varies from variety to variety in unpredicted manner. Low seed set in triploids are attributed to embryo mortality, deranged embryo - endosperm relations, irregular growth of pollen tube in styles of female flower and great variations in potency existing between pollens of different cultivars.Cultivated edible Musa species produce fruits through vegetative parthenocarpy[15], diploids like Calcutta 4, km5 and some tetraploids are prone to produce viable seeds due to even chromosomal number[20]. The unidentified plantain cultivar was found to produce numerous seeds up to 126 per bunch. This could be attributed to reversion in the field as was discovered by Irizarry et al.[9]. At Puerto Rico he found out that two major commercial cultivars that are of the horn type commonly cultivated in the area reverted to the French type where as the mother plant was false horn and that of the three bunch phenotypes resulting from their continued propagation depended on which section of the corm the buds originated. For instance that suckers originating from the completely mutated tissues produced the horn types while plants form the non-mixed tissue (mutated and none mutated) gave rise to instable plants producing horn type bunches.Seed production in triploids could also be attributed to some clonal variation from in vitro multiplied plants. Some clonal variation could be of great practical value in circumventing the extreme sterility of the false horn plantain in future.Some phenotypic variations were observed in the few germinated seeds when they were planted in the field. These morphological and genetic deviations were caused by the varying numbers of chromosomes of the parents which are diploid (AA) in km5, Calcutta 4 and triploid (AAB) in the plantains, AAA in Valery and ABB in Bluggoe.Assessment of black sigatoka diseaseThe assessment of black sigatoka disease was done and it shows that progenies 1, 2, and 4 are found to be partially resistant to black sigatoka disease while progenies 3 were resistant to the disease (Table 2). This means that progeny 3 was more superior to other progenies.When the hybrids were assessed for resistant to black sigatoka disease one[1] hybrid was found to be resistant while three[3] were partially resistant. Host response was determined at flowering by recording the number of standing leaves, the youngest leaf with symptoms, the youngest leaf spotted and the total leaf area attacked by BSD.Since all plantains traditionally grown by West and Central African farmers are susceptible to sigatoka disease [18], there varying host resistance must be introduced by the two wild sigatoka resistant parents Calcutta 4 and.Yangambi (km5). Calcutta 4 has a major gene for black sigatoka resistant (bsr) which is easily transferred to progenies through additive gene effect. The genes at the bsr locus may provide durable resistance to black sigatoka by slowing down disease development in the host plant.Host resistance is considered the most appropriate approach to sigatoka disease control because thousands of small—scale plantain farmers in Nigeria do not have access to and cannot afford the fungicides used by banana exporters and commercial producers. Genetic resistance is environmentally friendly and economically sustainable.From the trial carried out, it was established that seed production by the female parent was very low especially in cultivars pollinated. Some of the cultivars did not produce seeds such as USTPx02/01 and Valery. Nevertheless, plantain Musa species that are resistant to black sigatoka disease was produced. This new hybrid needs to be developed further so as to help in attacking black sigatoka disease in plantain.
In Table 1, it is evidenced that unknown plantain produced the highest seed of 126 followed by Calcutta 4. Others followed a decreasing order of seed production respectively. Seed setting in Musa genotypes is always difficult. This is partly due to chromosomal imbalance since most of the cultivated Musa species are triploids especially the plantains [18].Based on Table 1, Calcutta 4 and unknown plantain produced many seeds while Agbagba, Yangambi (km5), Bluggoe and French plantain produced very few seeds. Even some of the few seeds were found floating in water meaning that they were not viable. This is in line with Dessauw[2] report, that female fertility in French plantains is very low and absolute in horn type. In contrary to this, Swennen and Vuylsteke[20] working at IITA have identified 12 females fertile French plantains although they concluded that fertility varies from variety to variety in unpredicted manner. Low seed set in triploids are attributed to embryo mortality, deranged embryo - endosperm relations, irregular growth of pollen tube in styles of female flower and great variations in potency existing between pollens of different cultivars.Cultivated edible Musa species produce fruits through vegetative parthenocarpy[15], diploids like Calcutta 4, km5 and some tetraploids are prone to produce viable seeds due to even chromosomal number[20]. The unidentified plantain cultivar was found to produce numerous seeds up to 126 per bunch. This could be attributed to reversion in the field as was discovered by Irizarry et al.[9]. At Puerto Rico he found out that two major commercial cultivars that are of the horn type commonly cultivated in the area reverted to the French type where as the mother plant was false horn and that of the three bunch phenotypes resulting from their continued propagation depended on which section of the corm the buds originated. For instance that suckers originating from the completely mutated tissues produced the horn types while plants form the non-mixed tissue (mutated and none mutated) gave rise to instable plants producing horn type bunches.Seed production in triploids could also be attributed to some clonal variation from in vitro multiplied plants. Some clonal variation could be of great practical value in circumventing the extreme sterility of the false horn plantain in future.Some phenotypic variations were observed in the few germinated seeds when they were planted in the field. These morphological and genetic deviations were caused by the varying numbers of chromosomes of the parents which are diploid (AA) in km5, Calcutta 4 and triploid (AAB) in the plantains, AAA in Valery and ABB in Bluggoe.Assessment of black sigatoka diseaseThe assessment of black sigatoka disease was done and it shows that progenies 1, 2, and 4 are found to be partially resistant to black sigatoka disease while progenies 3 were resistant to the disease (Table 2). This means that progeny 3 was more superior to other progenies.When the hybrids were assessed for resistant to black sigatoka disease one[1] hybrid was found to be resistant while three[3] were partially resistant. Host response was determined at flowering by recording the number of standing leaves, the youngest leaf with symptoms, the youngest leaf spotted and the total leaf area attacked by BSD.Since all plantains traditionally grown by West and Central African farmers are susceptible to sigatoka disease [18], there varying host resistance must be introduced by the two wild sigatoka resistant parents Calcutta 4 and.Yangambi (km5). Calcutta 4 has a major gene for black sigatoka resistant (bsr) which is easily transferred to progenies through additive gene effect. The genes at the bsr locus may provide durable resistance to black sigatoka by slowing down disease development in the host plant.Host resistance is considered the most appropriate approach to sigatoka disease control because thousands of small—scale plantain farmers in Nigeria do not have access to and cannot afford the fungicides used by banana exporters and commercial producers. Genetic resistance is environmentally friendly and economically sustainable.From the trial carried out, it was established that seed production by the female parent was very low especially in cultivars pollinated. Some of the cultivars did not produce seeds such as USTPx02/01 and Valery. Nevertheless, plantain Musa species that are resistant to black sigatoka disease was produced. This new hybrid needs to be developed further so as to help in attacking black sigatoka disease in plantain.
|
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML