-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2013; 3(5): 67-72
doi:10.5923/j.plant.20130305.01
Interaction of Lectins Isolated from Four Selected Local Vegetables on Gastrointestinal Pathogenic Bacteria
Awoyinka O. A.1, Olajuyigbe O. O.2, Anyasor1, G. N.3, Osamika O.3, Adeniyi M.3
1Department of Medical Biochemistry, College of Medicine, Ekiti State University, Ado Ekiti, Nigeria
2Department of Biosciences and Biotechnology, Babcock University, Ilisan-Remo, Ogun State, Nigeria
3Department of Biochemistry, Ben Carson Snr., School of Medicine, Babcock University, Ilisan-Remo, Ogun State, Nigeria
Correspondence to: Awoyinka O. A., Department of Medical Biochemistry, College of Medicine, Ekiti State University, Ado Ekiti, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Lectins are carbohydrate recognizing proteins that bind mono- and oligosaccharide specifically and reversibly but are devoid of catalytic activity. In contrast to antibodies, they are not products of immune response. This study explores the isolation and partial characterization of lectin from leaves of Vernonia amygdalina, Talinum triangulare, Telfairia occidentalis and the kernels of Irvingia gabonensis focusing on their degree of antibacterial activities against some selected gastrointestinal pathogenic bacteria. Out of the different leaf materials, only lectin isolated fromVernonia amygdalina leaves exhibited the highest antibacterial activity against all tested bacteria. Agglutination of red blood cells by lectin from Talinum triangulare were significantly enhanced by D-lactose, D-maltose and D-glucose compared to lectin from other plant materials. Stability study showed that all the lectins were stable at temperature ranging from 20℃ to 30℃ and their activities were reduced with a corresponding increase in temperature. With the exception of lectin from Vernonia amygdalina, there were two different pH ranges for optimum activity of the respective isolated lectin. The study showed that the lectins from the different plant materials were capable of inhibiting the growth of the bacterial isolates. Thus, they may be suitable candidates for the prevention and treatment of gastrointestinal infections.
Keywords: Lectin, Hemagglutinating Activity, D-lactose, D-maltose, Gastrointestinal Infection, Vernonia Amygdalina, Talinum Triangulare, Telfairia Occidentalis, Irvingia Gabonensis
Cite this paper: Awoyinka O. A., Olajuyigbe O. O., Anyasor, G. N., Osamika O., Adeniyi M., Interaction of Lectins Isolated from Four Selected Local Vegetables on Gastrointestinal Pathogenic Bacteria, International Journal of Plant Research, Vol. 3 No. 5, 2013, pp. 67-72. doi: 10.5923/j.plant.20130305.01.
Article Outline
1. Introduction
- Recent progress in glycobiology has emphasized that numerous pathogens ranging from viruses and bacteria to pluricellular parasites use lectins as tools to recognize and bind to the oligosaccharides exposed by target cells and tissues[1]. While some dietary lectins have been known to preferentially facilitates the growth of gut bacteria such as Escherichia coli and Lactobacillus lactis[2], the pathogen surfaces have been known to bear a large number of oligoglicides that may be bound by specific lectins able to modulate the host infection[3-4] leading to acute gastrointestinal symptoms including nausea, vomiting, irritation and over-secretion of mucus in the intestines causing impaired absorptive capacity of the intestinal wall [5-8, 47]. Lectins are carbohydrate-binding proteins with remarkable ability to recognize and bind reversibly to carbohydrate moieties of complex glycoconjugates without altering the covalent structure of the bound glycosyl ligands[9]. In plants, these proteins play a protective role against predators[10-11]. Although some plant lectins are resistant to heat, heat processing can reduce their toxicity identified by consumption of food with high content of lectins while low temperature or insufficient cooking may be insufficient to completely eliminate them. In plants, legume lectins are the best know lectin family[12]. While leguminous seeds are particularly rich sources of lectin, lectins have also been isolated from other plant families and adopted for alternative therapy[13-14]. Consequentially, this study was aimed at investigating the antibacterial activities of lectins isolated from the leaves of Telfairia occidentalis Hook F. (fluted pumpmkin), Talinum triangulare (Jacq.) Willd. (water leaf), Vernonia amygdalina Del. (Bitter leaf) and the kernels of Irvingia gabonensis ( Ogbolo). The economic importances of these plants have been previously reported by various researchers[15-18], they indicated that these indegenous vegetables are highly reputed in traditional medicine. The herbal preparation of Telfairia occidentalis has been employed in the treatment of sudden attack of convulsion, malaria, anemia and the management of impaired immune defense[16-17, 19, 49]. Talinum triangulare (water leaf) is considered a cheap crop, easily cultivated and traditionally used as softener of other vegetable species. While Adewunmi and Sofowora[20] indicated the medicinal use of this plant in the management of cardiovascular diseases like stroke and obesity, its leaves extract is used as diuretic and in treatment of gastrointestinal disorder and hypertension[21]. Vernonia amygdalina (Bitter leaf) is eaten after being crushed and washed thoroughly to remove the bitterness[22]. All parts of the plant are pharmacologically useful. Among other uses, both roots and leaves are used in phytomedicine to treat fever, hiccups, kidney disease and stomach discomfort[23]. Antihelmitic, antimalarial properties as well as antitumourigenic properties have also been reported for extracts from this plant[24]. The Irvingia gabonensis, on the other hand, has been shown to play a significant role in weight management, control of blood glucose as well as blood cholesterol [25-27, 48]. Despite the aforementioned diverse pharmacological roles played by these vegetables in relation to various reports on their bioactive components[28], there have been less information on their lectin and its implication. Considering that there are not many data on their lectin the objective of this study was to investigate agglutination ability on pathogenic bacteria with the understanding that it would prevent their adhension to the gastro intestinal tract.
2. Materials and Method
2.1. Collection and Preparation of Plant Materials
- The leaves of Vernonia amygdalina, Talinum triangulare, Telfairia occidentalis and the dried kernels of Irvingia gabonensis were gotten from open from market in Ilishan-Remo, Ogun state, Nigeria. The leaves were cleaned by mild washing with disitlled water and were dried in the oven (Leader, UK ) at 45℃. Thereafter drying each respective sample were pulverized using a ceramic mortar and pestle and the resulting powdered forms of the vegetables were kept in a clean air-tight plastic container before analysis.
2.2. Partial Purification and Activity Assay of Lectin
- To isolate the lectin from the respective vegetables, the method of Awoyinka and Dada[29] was adopted. The pulverised samples were dissolved in water in ratio 1:20 centrifuged at 1500 revolution per minutes (rpm) for 30minutes. The pellets were discarded and supernatant collected for ammonium sulphate precipitation as described by Trowbridge (30). The precipitated protein were pulled together and dissolved in 240ml of distil water, the resulting mixture was concentrated by ultra filtration (Millipore, India) at 1500g for 30minutes before dialyses against 0.15M NaCl – 0.01M NaPO4 buffer for 24hours. The dialysed sample was further purified by anion exchange affinity chromatography method using 2g DEAE- Sephadex A-50 previously dissolved in 50ml of 0.15M NaCl – 0.01M NaPO4 buffer before equilibration in a column (8.5 × 1.5cm). Thereafter, the column was washed with the same buffer and loaded with dialyzed precipitated protein and eluted with 0.2M glucose solution. The eluents were assay for lectin using hemagglutination activity as marker. Agglutination of the red blood cells by the crude extract and the various fractions that were obtained during purification was estimated as described by Bing et al.,[31]. This involves a serial two-fold dilution of the lectin solution of human erythrocytes in phosphate buffered saline (pH7.2) at room temperature to allow for agglutination of the erythrocytes to take place. The hemagglutination titre of the lectin expressed as the reciprocal of the highest dilution exhibiting visible agglutination units were obtained. The total protein content was determined using the biuret test[32].
2.3. Anti-Bacterial Assays
- The in vitro sensitivity of the bacteria isolates - Pseudomonas aeruginosa ATCC 19582, Shigella sonnei ATCC 29930, Proteus vulgaris ATCC 6830, Staphylococcus aureus ATCC 6538, Escherichia coli ATCC 25922, Klebsiella oxyteca, Salmonella typhi ATCC 13311, Proteus mirabilis, Vibrio cholerae, and Entamoeba faecalis to the test purified lectin was performed by the disc diffusion method[33]. The test bacterial isolates were inoculated over the entire surface of freshly prepared Mueller Hinton Agar (MHA) in a 9 cm petri dish. Two sterile paper discs (3 mm each) were placed on the opposite sides of the plate containing the growing colony. The sterile paper discs were first moistened with 10 µl of the purified lectin using a micropipette before being transferred into the inoculated plates with heat sterilized forceps. The plates were kept for 30 min prior to incubation. Two control plates were also maintained in each case. One plate received only sterile water on the disc while the other received 1M buffered saline before being incubated for 24 h at 37℃. The antibacterial activity of the lectins was determined by measuring the mean diameter of the zones of inhibition in millimeter with transparent meter rule.
2.4. Test of Hemagglutination Inhibition by Various Carbohydrates
- The hemagglutination inhibition of lectin-induced by sugars at different concentrations of 25, 50, 100, 200, 400, and 800 mM were performed as described by Kuku et al.,[34]. Serial two-fold dilutions of sugar samples were prepared in phosphate buffer saline. All the dilutions were mixed with an equal volume (50 µl) of the lectin solution of known hemagglutination units. The mixture was allowed to stand for 1 h at room temperature and then mixed with 50 µl of 4% human erythrocyte suspension. In this study, the sugars used include sucrose, lactose, maltose, fructose, glucose and galactose.
2.5. Effect of Temperature on Hemagglutinating Activity
- The effect of temperature on the agglutination activity of lectin obtained from Bitter Leaf and Water leaf were determined by carrying out assays at different temperatures as described by Patrick et al.,[35]. The purified lectin were incubated in a water bath for 30minutes at 10℃, 20℃, 30℃, 40℃, 50℃, 60℃, 70℃, 80℃, 90℃ and 100℃ and then cooled to 20℃. Hemagglutination assay was carried out as previously described.
2.6. Effect of pH on Hemagglutinating Activity
- The effects of pH on the activity of the lectin from the vegetables were determined by incubating the respective lectins in 0.15M NaCl-0.01MNa3PO4 buffer at pH: 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12. The various pH values were obtained by altering the buffer with concentrated HCl and 1M NaOH and assaying for haemagglutination activity.
3. Results and Discussion
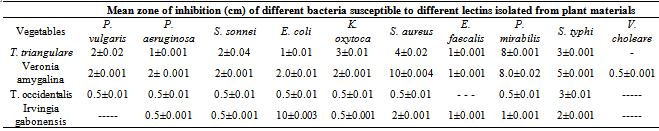
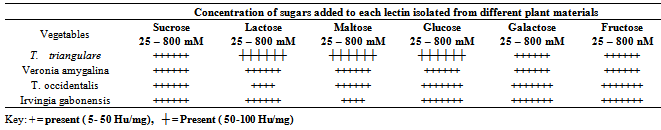
- In this study it was discovered that all the vegetables contained a measurable amount of hemagglutinating protein (lectin) with a peak value of 208 HU/mg. These lectins agglutinate the human red blood cells of the ABO system at approximately the same dilution (non-specifically and fairly strongly). Similarly lectin from the seeds of Dioclea reflexa and Capsicum annum non specifically agglutinates the erythrocytes of ABO human blood groups. These lectins are referred to as non- specific lectins[36-38]. The antibacterial assay showed that all the lectins isolated from the different vegetables exhibited varied aggulutination abilities to each of the bacteria. Lectin isolated from Veronia amygdolina was the most active. It effectively inhibited all the test bacteria. The most inhibited bacteria included Escherichia coli, Staphylococcus aureus, Proteus mirabilis and Salmonella typhi while the most resistant bacteria were Vibrio cholerae, Salmonella typhi, Entamoeba faecalis and Proteus vulgaris respectively. With the exception of lectin from Vernonia amygdalina, lectins isolated from other vegetables did not inhibit the growth of Vibro cholera. While lectin from Irvingia gabonesis failed to inhibit the growth of Proteus vulgaris, Talinum triangulare was unable to inhibit Salmonella typhi, and that of T. occidentalis was unable inhibit Entamoeba faecalis. In this study, the antibacterial activities of lectin extract from these vegetables indicated their potential ability tocontrol the selected gastrointestinal pathogenic bacteria. In agreement with this study, Oboh et al.,[39] and Paiva et al.,[40] earleir reported that N-acetyl-D-glucosamine binding lectin strongly inhibited the growth of Staphylococcus aureus, Pseudomonas aeruginosa, Streptococcus spp. and Klebsiella spp. Laura[8] indicated that bacteria could bind dietary lectins bound to cell membrane surfaces to increase internalization of surface bound enterotoxin There was varying degree of hemagglutination at the sugar concentrations on all the isolated lectin in this study. As shown in Table 1 except from Talinum triangulare, lectin isolated from other vegetables showed a slight appreciable hemagglutination activity at the range of 5 - 50 Hu/mg when the carbohydrates- sucrose, maltose, glucose, galactose and fructose were added progressively from 25 – 800 mM. There was inhibition of hemagglutination activity at concentration of 25 – 100 mM when lactose and maltose were added to lectin from Telfairia occidentalis and Irvingia gabonesis respectively but hemagglutination was observed at 5 - 50 Hu/mg when the sugar was increased to 400 mM. Hemagglutination activity was recorded at higher range of 50 - 100 Hu/mg when the various concentrations of lactose, maltose and glucose were added to lectin isolated from Talinum triangulare but slightly showed hemagglutination activity similar to that of other vegetables when sucrose, galactose and fructose were added progressively from 25 – 800 mM. These findings suggested that all the sugars did not inhibit the activity of the lectin found in the vegetables. Thus, they might be free sugar binding lectins as described by Okamoto et al.,[41] in their report on numerous marine algal lectin such as Gracilaria bursa-pastoris. The presence of these sugars in the diet would still probably enable the lectin under study to prevent adhension of the pathogenic bacteria at the gastro intestinal tract.
|
|
4. Conclusions
- In this study, the ability of lectins from vegetables to inhibit the growth of selected microorganisms could justify their potential ability in controlling bacteria capable of causing diseases in man and animals. On this note it is sufficient to say since lectins are not only readily available but offer higher degree of specificity with carbohydrate moieties, they can be useful as diagnostic markers as against the use of monoclonal antibodies which is expensive and time consuming. They could be incorporated into drug designs as well as subjected to clinical scrutiny as it has the potential of replacing synthetic raw materials in the treatment of bacterial infections.
ACKNOWLEDGEMENTS
- We want express our gratitude to invaluable support of Mrs Akeredolu Bola and Mr. Omeonu of Babcock University Teaching Hospital for their technical assistance during thecourse of this research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML