-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2012; 2(6): 195-198
doi: 10.5923/j.plant.20120206.04
Influence of Aclonifen on the Growth of Rhizobium Phaseolii and the Yield of Green Beans (Phaseolus Vulgaris L.)
Orhan Vedat Gürsoy 1, Hüseyin Padem 2
1Mugla University, Milas Vocational College, Department of Technical Programing, 48200, Mugla, Turkey
2International Burch University, Faculty of Engineering, Department of Genetics and Bioengineering, Francuske revoluciye, 71000, Sarajevo, Bosnia and Herzegovina
Correspondence to: Hüseyin Padem , International Burch University, Faculty of Engineering, Department of Genetics and Bioengineering, Francuske revoluciye, 71000, Sarajevo, Bosnia and Herzegovina.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
In this study, the degree of the negative effect of Aclonifen containing herbicide on Rhizobium phaseolii, total mesophilic bacteria (TAMB), yeast and molds (YM), the yield and correlation among parameters of bean under natural field conditions were investigated. Rhizobium phaseolii stock culture (8.71 log cfu/g), was mixed with the media in pots homogenously at a dose of 0, 1 and 2 g. When the young bean plants reached the 5-6 true leaf stage 600 g/l Aclonifen containing Challenge 600, was applied as a herbicide at the dose of 0, 625, 1250, 1875 and 2500 ml/ha, respectively. The effect of the Rhizobium and herbicide treatments on Rhizobium phaseolii, TAMB, YM and the bean yield were tested. The results obtained from the trial revealed that the number of Rhizobium bacteria, TAMB, YM and also the yield were reduced by the increased herbicide dose. The number of TAMB and YM were not affected by Rhizobium treatments but yield was.
Keywords: Aclonifen, Mesophilic Bacteria, Molds, Rhizobium phaseolii, Yeast
Cite this paper: Orhan Vedat Gürsoy , Hüseyin Padem , "Influence of Aclonifen on the Growth of Rhizobium Phaseolii and the Yield of Green Beans (Phaseolus Vulgaris L.)", International Journal of Plant Research, Vol. 2 No. 6, 2012, pp. 195-198. doi: 10.5923/j.plant.20120206.04.
Article Outline
1. Introduction
- Beans (Phaseolus vulgaris L.), with their high percentage in the share of vegetable production in Horticultural activity, play a great role in many countries. Even despite great variations in the climate conditions, they can be grown all over, especially in semiarid countries[19]. In Turkey, they occupy an area of 98.2 hectares and 154.000 tons of dried beans and 563.000 ton fresh beans are produced per year[3]. The crop can be consumed either as a fresh vegetable when the pods are green or when the seeds are completely matured. Dried beans are a rich source of protein and agronomists desire to maximize their production. Since snap beans are produced mainly under irrigated conditions together with manure application to the fields, this may cause intensive weed emergence and growth. Then hand hoeing and the other means of mechanical controls are practiced.During the growth of the plants, the amount and activity of nitrogen fixing bacteria is very important and, in addition to weeds, are one of the most important pests which must be controlled. In dry bean production, Imazamox shows potential as a post-emergence weed control[6]. At low densities, either mechanical tillage or herbicides alone were effective but at higher densities herbicides combined with mechanical tillage were required for effective control[2]. Pre-plant incorporated and pre-emergence application of Imazethapyr alone or in a tank mixture with S-metolachlor at low and high rates did not have any significant effect on plant height, dry weight, seed moisture content or yield but caused crop injury. The higher rates caused higher crop injury both alone or in tank mix application. PPI aplication caused less crop injury than pre-emergence application[16].Imazamox+fomesafen mixture caused significant visual injury and tended to decrease lima bean height and yield. Despite some initial injury observed in the metolachlor, Imazethapyr applied pre at 75 g/ha and quizalofop-p applied post at 72 g/ha have excellant potential as weed management tools[13]. Herbicides registered for Lima Beans (Phaseolus lunatus L.) do not constantly control many troublesome weeds. Some herbicides registered for soybeans (Glycine max) will control these weeds but their tolerance in Lima beans is not known. Cloransulam, flumetsulan, metolachlor,sulfentrazone, lactofen, imazergapyr applied alone or in a mixture, low crop injury observed with cloransulam and Imazethapyr plus metolachlor. All others caused some injury but lactofen was the highest[5]. Many researchers did not pay too much attention to nitrogen fixing bacteria.During the selectivity tests of new compounds, many points have been taken into consideration. However, at the beginning of the field application herbicides will be absorbed by weed roots as well as crop roots. Though some slight stress may be observed in the crops even if the herbicide is accepted as selective, this situation could be neglected since the crops may recover quickly. Either light or medium damage may cause some delay in the growth of the crop, too. Earlier studies have demonstrated the adverse effects of some kinds of herbicides on Rhizobium growth and its symbiosis. Insufficient information is available on the effect of herbicides on Rhizobium, total mesophilic bacteria, yeast and moulds on some legumes.
2. Main Body
2.1. Materials and Methods
- The study was carried out at Suleyman Demirel University Agricultural Research and Experimental Station. The 18 liter capacity plastic bags were filled with the media prepared as a mixture of manure, loamy soil and sand at a ratio of 1:1:1[15]. at a total of 15 liters of each. In order to provide the natural environment, the filled bags were buried in the field to keep both the soil and plastic bags surface at the same level.Before the application of Rhizobium phaseolii, the microbiological properties of the media were determined as total mesophilic bacteria, (TAMB), 6.63 log cfu/g, yeast and molds (YM): 4.30 log cfu/g and Rhizobium phaseolii 1.40 log cfu/g.Rhizobium phaseolii stock culture (8.71 log cfu/g), produced by Soil and Fertilizer Research Institute in Ankara-Turkey, was mixed with the media in pots homogenously at a dose of 0, 1 and 2 g. Two seeds of the Romano bean variety were sown in each pot on June 5th. This variety of beans is widely produced in Turkey for fresh bean consumption. The planting distance was adjusted to 50 cm between rows and 25 cm between plants.When the young bean plants reached the 5-6 true leaf stage[4]. 600 g/l Aclonifen containing Challenge 600 were applied as a herbicide at the dose of 0, 625, 1250, 1875 and 2500 ml/ha, for the first time. The surface area of the pots was 962 cm2 The generally recommended dose should have been 1250 ml/ha. The field trial[8] was designed and carried out according to split plot design with four replications. Each replication consisted of four pots and 8 plants.During the growing period with 30 days interval three times 20 g soil samples were taken from each pot starting from 17th of July in order to determine the quantity of Rhizobium, TAMB and YM. Each time the samples were taken in the morning 24 hours after each irrigation.
2.2. Microbiological Analyses
- The sterilized jars were used for the soil samples. The 10 g of soil samples mixed with various concentrations of Rhizobium phaseolii and herbicide were added in 90 ml NaCl solution (0.85 %) and diluted up to 10-5.The total aerobic mesophilic bacteria (TAMB) were enumerated on Plate Count agar (Merck, Darmstadt, Germany). The number of yeasts and molds (YM) was determined in Potato Dextrose agar (Merck, Darmstadt, Germany) reduced to pH 3.5 with tartaric acid. Rhizobium phaseolii counts of the soil samples were enumerated on Mannitol Yeast Extract agar and incubated at 25℃ for 3-4 days[17].The microbial counts were determined as colony forming units (cfu) in gram of the samples. The results of microbiological analyses shown as log cfu/g. All analyses were formed in duplicate.
2.3. Findings
2.3.1. The Effects of Aclonifen Treatments on R. Phaseolii
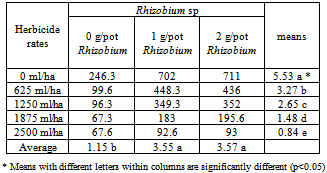
- Bean plants grown with different doses of herbicide and R. phaseolii stock culture based on the results shown on the Table 1. Rates of Aclonifen adversely affected the R. phaselii counts. It was determined that the number of Rhizobium bacteria in the media were reduced with the increased doses of herbicides. The interactions of HerbicidexRhizobium were significant at the 0.01 level. At the same time the two doses of Rhizobium were not significant between themselves but the control was significantly different.
|
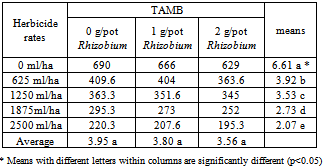
2.3.2. The Effects of Treatments on TAMB
|
2.3.3. The Effect of Treatments on YM
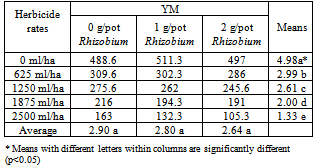
- It is statistically determined that the number of YM was not affected by the Rhizobium treatments. However the effect of the interreaction of the HerbicidexRhizobium on YM numbers in media was statistically significant at the 0.01 level. The number of YM was high on non-treated check pots but it was the lowest on the fourth dose of the herbicide application. It is clear that increased herbicide dose reduced the number of YM in the media.
|
|
|
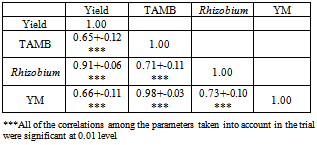
2.3.4. The Effects of Treatments on Yield and Correlation among Parameters
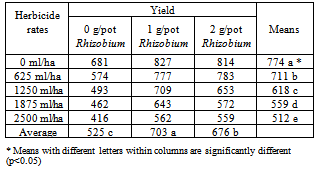
- The highest yield was obtained from the 1 g/pot Rhizobium treatment. This was followed by the 2 g/pot treatment and the lowest yield was obtained from the check pots. As can be clearly seen from the table that the herbicide application reduced the yield. The highest yield was obtained from the check pots. The differences of either herbicides or Rhizobium application were significant at 0.05 level. Also the effect of interreaction between Herbicidex Rhizobium was significant on the yield.
2.4. Results and Discussion
- Since herbicides are necessary to achieve maximum yield, their influence on nodulation may conflict with the crop managements[10]. Herbicide application is very common in bean production. It is well known that the bean plants can get most of their nitrogen needs mainly by the nodules formed on their roots from the nitrogen in the atmosphere. Many of the chemicals, e.g. pesticides, may be potentially hazardous and associated with symbiotic nitrogen-fixingmicroorganisms[12]. The inoculated seed has to provide the incoming rhizosphere with enough microorganisms to nodulate and fix nitrogen[14].Based on the results obtained from this trial, it was found that the application of aclonifen had a reducing effect on Rhizobium bacteria together with TAMB and YM. There is a probability that Aclonifen may have a toxic effect on Rhizobium, TAMB and YM population existing in the bean producing field. Toxicity of Aclonifen to Rhizobium, TAMB and YM increased progressively with increase in rates of herbicide. Similar results were obtained by Ahemad and Khan (2009) that quizalafop-p-ethyl and clodinafop proved to have a lethal effect on symbiotic properties.Weeds cause an 8.7 % reduction in bean production in the USA[7]. The severe competition for nutrients light and water between crops and weeds at early stage requires pre-emergence herbicide application. Among the many herbicides used for weed control in green bean crops, Aclonifen has proved to be effective against a wide spectrum of broad-leaved weeds in legumes[18].Compared to check pots, the population of Rhizobium increased in the Rhizobium applied pots. However there were no differences in terms of Rhizobium population between 1 g/pot and 2 g/pot Rhizobium application. Also Rhizobium application had no effect neither on the TAMB nor YM populations. The infective phase of the symbiosis begins just before the contact occurs between bacteria and root hairs[9] and during this period, the association is highly sensitive to the soil environment[11].
3. Conclusions
- Both the Rhizobium and herbicide application considerably affected the bean yield. The highest yield was obtained from 1 g/pot Rhizobium application. Increased herbicide application reduced the yield. Very important correlations were determined among all the parameters evaluated in this research. These findings are probably due to the quick inactivation of Aclonifen in growing media.As a result of this research, it was found out that the application of aclonifen for controlling weeds in bean production had a negative effect on the soil microbiology. The application of Aclonifen may only be recommended as a last resort for weed control in bean production. The trial was carried out under natural field conditions. The same type of trial may also be carried out in a laboratory to find out the effect of Aclonifen on the soil microbial activity.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML