-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2012; 2(5): 151-159
doi: 10.5923/j.plant.20120205.03
Effect of Saponin Extracted from Passiflora alata Dryander (Passifloraceae) on development of the Spodopterafrugiperda (J.E. Smith) (Lepidoptera, Noctuidae)
Marianna Pilla D’Incao 1, 2, Greice Gosmann 2, Vilmar Machado 1, Lidia Mariana Fiuza 1, Gilson R. P. Moreira 2
1Laboratório de Microbiologia e Toxicologia Universidade do Vale do Rio dos Sinos , UNISINOS, Av. unisinos, 950 CEP 93022-000, São Leopoldo, RS. Brasil
2Instituto de Biociências, Universidade Federal do Rio Grande do Sul, UFRGS. Av. Ipiranga, 2752, 90610-000, Porto Alegre, RS, Brasil
Correspondence to: Vilmar Machado , Laboratório de Microbiologia e Toxicologia Universidade do Vale do Rio dos Sinos , UNISINOS, Av. unisinos, 950 CEP 93022-000, São Leopoldo, RS. Brasil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Thesaponins are glycosides with wide distribution among plants which may be toxic for herbivorous arthropods. Their insecticidal activity may be associated to the ability of producing alterations in the feeding behavior, in the molting process and causing death. This study evaluated the lethal and sub lethal effects of a saponins extract, obtained from Passifloraalata on Spodopterafrugiperda, by ingestion tests with artificial diet. Nine solutions with increasingextract concentration and a control solution with sterile distilled water were used in the test. In total 1500 insects were used in 5 repetitions, 30 replicates per concentration. Considering the total mortality, all treatments differed statistically from the control one, but not among them. Most of the observed effects were sub lethal, in which 68.3% insects presented deformation. The number of deformed insects per treatment increased as the extract of saponins concentration increased. In conclusion the mortality revealed significant difference between control and other treatments, which indicated the potential of saponins present in P. alata as a control agent of S. frugiperda. This potential needs to be better evaluated, especially if the facilities of raising and large scale production of the plant are considered.
Keywords: Saponin, Spodopterafrugiperda, Lethal Effects, Fall Armyworm, Passifloraalata
Article Outline
1. Introduction
- The researchesdesigned at identifying new toxic substances in order to control agricultural plagues are constant in the activities related to management and integrated control of plagues nowadays. This is partially a resultof the knowledge of the negative impactof the chemical products used in the ecosystem as well as the possibility of evolution of the resistance of the target insects to the products used, whether these are chemical and/or biological. A great number of these researches aredirected to the evaluation of toxicity of secondary metabolite of plants, such as phenols, alkaloids, glucosinolates, cyanogenic glycosides and saponins.1More than 2000 species of plants are known to possess some insecticidalactivity. In many cases plants have a history as traditionalmedicines or are used to kill or repel insects. In spite of the various studies available about the entomotoxic effects of extracts of different species of plants, their application is still incipient.2The saponins are glycosides with detergent properties, due to the presence of hydrophobic (aglycones) and hydrophilic (sugar) components with wide distribution among plants which may be toxic forherbivorous arthropods such as mites, beetles and Lepidoptera, among others.3-7Their insecticidal activity may be associated to the ability of producing alterations in the feeding behaviour, in the molting process, of interacting with hormones that regulate the growth and causing death in the different stages of development.8-11Their wide spectrum of action reaches a great number of herbivores and its amplitude of physiological impacts makes these substances an excellent model for the study of the effect of natural substances with insecticidal activities.8The passion fruit,Passifloraalata, is one of the main species of economic importance of the gender cultivated in Brazil, which is used mainly as fruit and for juice and sweets production as well as a medicinal plant.12-16Thesaponins are the main substances of its secondary metabolism. Five types of saponins were isolated and identified in its leaves: one is a steroid and four are triterpenic(3-6).17-18The fall armyworm Spodopterafrugiperda (J. E. Smith) (Noctuidae) is a polyphagous insect, using more than 50 species of plants as food supplies, distributed in over 20 families,19 predominantly in grasses, among them corn and rice, which are important cultures for Brazilian and world economy. In rice,S. frugiperda is considered a pestof the initial phase, since it attacks the seedlings in the beginning of their development, feeding on the leaves and cutting the new stalk close to the ground,20causing losses of 14 to 24% of the grains. Depending on the population level, the destruction of the crop can be total.21In maize, the attack can occur from the seedling stage until the tasseling and the formation of spikes. In the later attacks specimens between the stalk and the cob can be found, where they penetrate the female inflorescence destroying the grain.22The effect of saponins extract of some plants on the development of the Spodopteraspecieswas demonstrated in studies with S. litura.2,4,23-27The results of these studies indicate alterations in the feeding behaviour, lengthening of the larvae and/or pupa stages, increased mortality and reduced fertility. The study of plants with potential insecticidal activity is important to discover new active molecules, which may be isolated from plants or synthesized, or a molecule-prototype for structural changes to obtain a more active compound.This work evaluated the effects of saponins extracts of PassifloraalataonSpodopterafrugiperda, identifying the sub lethal and lethal effects of these substances in different development stages of this species.
2. Material and Methods
2.1. Preparation and Characterization of the Saponin Extracts
- To obtain saponin extract the dried leaves were submitted to maceration with 70% ethanol. The ethanol was filtered and placed in a rotary evaporator; the aqueous residue was extracted successively with chloroform, ethyl acetate and n-butanol. The evaporation of n-butanol fraction resulted in a fraction consisting mainly of saponins, which will be called henceforth saponin extract.17Thesaponin extract is characterized as a yellow-orange powder.
2.2. Obtention and Rearing of Insects
- The establishment of S. frugiperda began with immaturelarvae collected in rice plantations, with the collaboration of researchers at the Estação Experimental do Arroz (EEA) of the InstitutoRiograndense do Arroz (IRGA), located in Cachoeirinha, RS. The insects were reared in the room for the creation of insects of the Laboratory of Microbiology of the University of Vale do Rio dos Sinos (UNISINOS), heated to 26°C, 75% RH and 12h photo phase. The adults were kept in plastic cages, fed with a glucose solution of 10%. Eggs were collected three times a week and put in gerbox with diet. Five or six days after hatching, the larvae were individualized in plastic cups, and maintained with artificial Poitoutdiet,28 where they remained until they became pupae. As a routine procedure adopted, the pupae were identified, separated by sex, kept in sleeves with moistened filter paper and covered with tulle, until emergence of adults.
2.3. Bioassay
- For the test with S. frugiperda nine solutions with increasing concentrations of saponin extract were used due to the 2x (312, 625, 1250, 2500, 5000, 10000, 20000, 40000 and 80000ppm) and a control of sterile distilled water. A solution of 20ml was prepared for each concentration, which, after use, was stored at 4°C. In this case, the extracts were diluted in sterile distilled water. The second instar larvae of S. frugiperda were maintained for seven days in mini acrylic plates (35mm diameter) containing Poitout diet, where 100μL of the solutions were applied on the diet. After thisthey were transferred to plastic cups containing only artificial diet, where they were observed until emergence of adults for the evaluation of possible sub lethal effects (Figure 1). Damages were evaluated under stereoscopic microscope and classified according to intensity (low, medium and high) (Figure 2).Bioassays were kept at 26°C, 75% RH and 12h photo phase. Five repetitions of 30 larvae/concentration, totalizing 1500 insects were performed.
2.4. Data Analysis
- Data were tested for normality using the chi-squared and Shapiro-Wilks and the homogeneity with the tests of Hartley and Bartlett. When these criteria were satisfied, they were compared by an ANOVA followed by multiple tests of Tukey. Otherwise, they were compared by Kruskal-Wallis followed by Dunn's multiple test. Statistical analysis was performed with the aid of TOXTAT Software version 3.3.29To compare mortality rates between larvae and pupae of S. frugiperda the Student t test was conducted for different means and to analyse the deformation of the same species a simple linear regression was made.30
3. Results
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
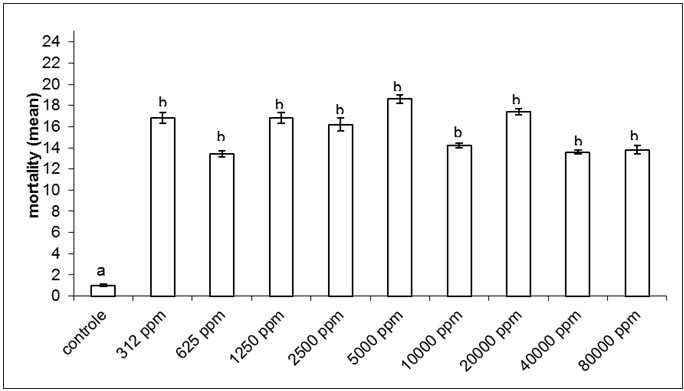 | Figure 3. Total mortality (mean ± standard error) of Spodopterafrugiperda after bioassay of ingestion in different concentrations of saponin.Columns followed by different letters are significantly different (ANOVA, followed by Tukey's multiple tests α=0.05) |
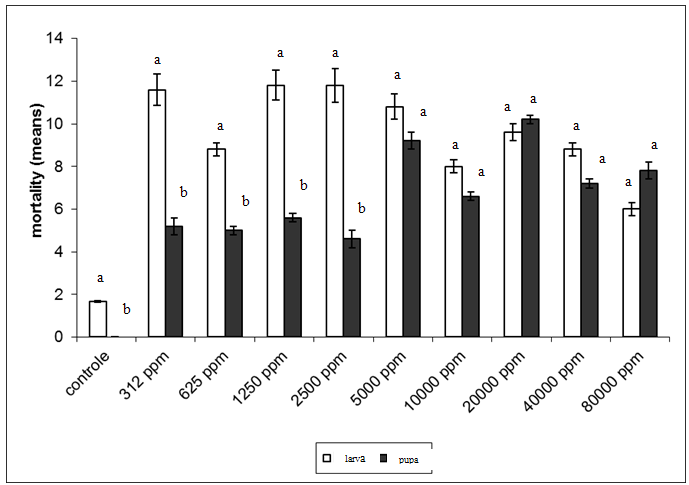 | Figure 4. Mortality of larvae and pupae of Spodopterafrugiperda after ingestion test of saponin at different concentrations. Columns followed by different letters differ significantly at a given concentration (Student's t test, α = 0.05) |
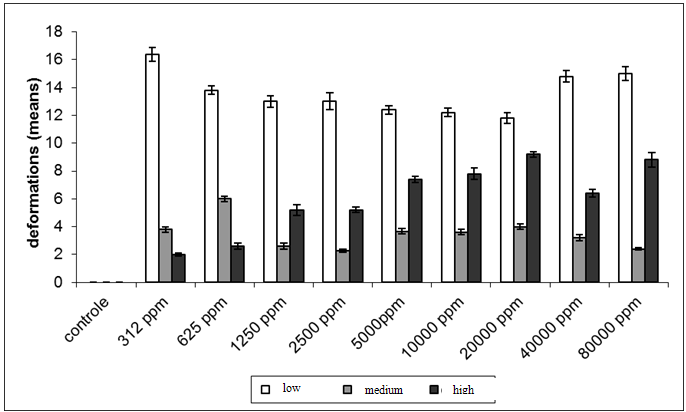 | Figure 5. Deformations (mean ± standard error) observed in Spodopterafrugiperda, after ingestion test of saponin, divided into three categories (low, medium and high), by concentration of saponin |
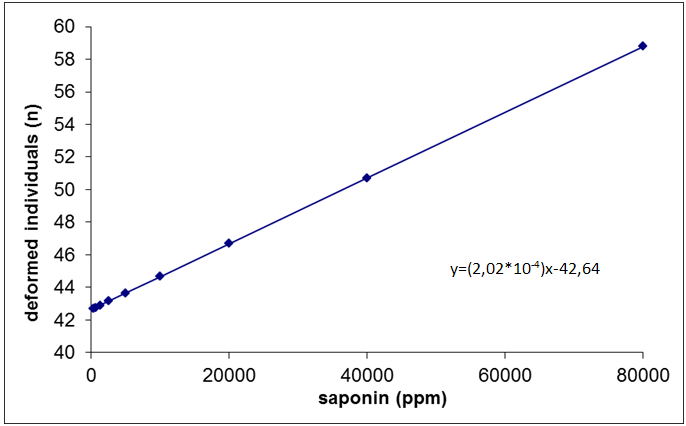 | Figure 6. Linear regression of the number of deformations observed in Spodopterafrugiperda after ingestion test of different concentrations of saponin |
|
4. Discussion and Conclusions
- This study identified the impact of saponins extract on the different stages of development of S.frugiperda.Besides the mortality of larvae and pupa, the presence of structural deformities was observed in all stages. When considered the total impact of the treatments 47.4%of the adults emerged without structural problems identified by optical microscopy. The effects of the saponin extract on S. frugiperdamay be associated to a deterrent toxic action and/or by its interactions with substances responsible for the different stages of development of the specie.The deterrent effect reduces the consumption of food producing nutritive deficiency causing deficient growth or deformities, which may lead to death or inhibit the progress for the next stage.26,27,31,32Among the alterations observed, the presence of necrosis points stood out, sclerotinization problems and retention of larvae characters and tumors.These results are similar to the ones registered,21for the biotypes of corn and rice of S. frugiperdausing different extracts of different species of plants.It is important to highlight that alterations in the length of the larvae and pupal stages were not observedin this study, as registered for S.littoralis23e for S. frugiperda.33The differences in these results may be associated to the variation in the composition of saponins among the plant species used for the acquisition of the extracts.3,34The physiological basis of the toxicity of saponins has not been fully elucidated, but we know that there is a great interaction between them and the cell membranes, with effects on the hydrophobic-lipophilic balance and permeability of these because they are capable of forming complexes such as sterols, for instance, cholesterol.10,11,35The toxicity of saponins on arthropods may derive from their ability of interacting with free steroids of the intestine and/or of inhibiting the digestive proteases, reducing the rates of digestion and absortion.8,35,36The mortality and deterrence caused by saponin were also observed in experiments that evaluated the effects of different types of saponin for aphids,38,39,42nematodes,40-42beetles31,43,44 and Spodoptera sp.2,5,6,25,27A fact that drew attention was the appearance of specimensin the intermediary period between larvae and pupae (1.8% of exposed organisms). Apparently, the organisms began the process of becoming pupae and could not complete it, which led to death. The appearance of intermediate individuals between pre-pupae and pupae can occur when the activity of juvenile hormone, which controls the metamorphosis, is affected.32Furthermore, the metamorphosis may have been affected because of saponins form complexes with sterols, like cholesterol, both in the intestine in cell membranes of insects. These complexes formed by saponin make sterols unavailable, influencing the synthesis of ecdison, one of the hormones involved in the process of molt. It has been shown that insects are unable to produce these sterols, removing them entirely from the diet.10,11,34,36,39 The statements of these authors may account for the large number of deaths of larvae and pupae during the processes of molt and emergence of pupae malformed, beyond the failures of sclerotinization observed.In conclusion the mortality of S. frugiperda showed significant difference between control and other treatments. It was observed that the death of the specimens was not immediate after the ingestion of saponin, but slow and gradual, which apparently occurred under the influence of saponin in the metabolism of the larvae, pupae and adults. These results indicate the potential of saponins present in P. alataas a control agent of S.frugiperda. This potential needs to be better evaluated, especially if the facility of raising and large scale production of the plant is considered.
ACKNOWLEDGMENTS
- We acknowledge the CNPq by the financial support to do this research at Biology Postgraduate Program of UNISINOS
References
| [1] | Tagliari MS, Knaak N and Fiuza LM. Plantas inseticidas: interações e compostos. PesquisaAgropecuáriaGaúcha 10: 101-111, 2004. |
| [2] | Ulrichs C, Mewis I, Adhikary S, Bhattacharyya A and Goswami A. Antifeedant activity and toxicity of leaf extracts from PorteresiacoarctataTakeoka and their e Vects on the physiology of Spodopteralitura (F.). J Pest Sci 81: 79-84, 2008. |
| [3] | Vincken JP, Heng L, de Groot A and Gruppen, H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry 68: 275-297, 2007. |
| [4] | Kamaraj C, Rahuman AA and Bagavan A. Antifeedant and larvicidal effects of plant extracts against Spodopteralitura (F.), Aedesaegypti L. and Culexquinquefasciatus Say. Parasitol Res 103: 325-331, 2008. |
| [5] | Park HY, Kim JY, Kim HJ, Lee GH, Park JI, Lim CW, Kim YK and Yoon HD. Insecticidal and Repellent Activities of Crude Saponin from the Starfish Asteriasamurensis. J Fish SciTechnol 12:1-5, 2009. |
| [6] | Saha S, walia S, Kumar J, Dhingra S andParmar BS. Screening for Feeding Deterrent and Insect Growth Regulatory Activity of TriterpenicSaponins from Diploknemabutyracea and Sapindusmukorossi. J Agric Food Chem 58: 434-440, 2010. |
| [7] | Prasifka JR, Bradshaw JD, Lee ST and Gray ME. Relative Feeding and Development of Armyworm on Switchgrass and Corn, and Its Potential Effects on Switchgrass Grown for Biomass. J Econ Entomol 104:1561-1567, 2011. |
| [8] | IKBAl I. Saponins as insecticides: a review. Tunisian Journal of Plant Protection 5: 39-50, 2010. |
| [9] | De Geyter, E, Lambert E, Geelen D and Smagghe G. Novel advances with plant saponins as natural insecticides to control pest insects. Pest Technol 1: 96-105, 2007. |
| [10] | De Geyter E, Smagghe G, Rahbé Y and Geelen D. Triterpenesaponinsof Quillajasaponaria show strong aphicidal and deterrent activity against the pea aphid Acyrthosiphonpisum. Pest ManagSci DOI:10.1002/ps.2235, 2011. |
| [11] | De Geyter E, Swevers L, Soin T, Geelen D and Smagghe G. Saponins do not affect the ecdysteroid receptor complex but cause membrane permeation in insect culture cell lines. J insect physiol 58:18-23, 2012. |
| [12] | Cervi AC. O gênero Passiflora L. (Passifloraceae) no Brasil, espécies descritas após o ano de 1950. Adumbrationes ad SummaeEditionem 16: 1-5, 2006. |
| [13] | Reginatto FH, De-Paris F, Petry RD, Quevedo J, González Ortega G, Gosmann G and Schenkel EP. Evaluation of anxiolytic activity of spray dried powders of two south Brazilian Passiflora species. Phytother Res 20: 348-51, 2006. |
| [14] | Mello FB, Langeloh A and Mello JRB. Toxicidade Pré-Clínica de Fitoterápico Contendo Passiflora alata, Erythrina mulungu, Leptolobiumelegans e Adonisvernalis. Lat Am J Pharm 26:191-200, 2007. |
| [15] | Barbosa PR, Valvassori SS, Bordignon CL, Kappel VD, Martins MR, Gavioli EC, Quevedo J and Reginatto FH. The aqueous extracts of Passifloraalata and Passifloraedulis reduce anxiety-related behaviors without affecting memory process in rats. J Med Food 11: 282-8, 2008. |
| [16] | Gosmann G, Provensi G, Comunello LN and Rates SMK. Composição química e aspectos farmacológicos de espécies de Passiflora L. (Passifloraceae). Rev Bras Biocien 9: 88-99, 2011. |
| [17] | Reginatto FH, Kauffmann C, Schripsema J, Guillaume D, Gosmann G and Schenkel EP. Steroidal and TriterpenoidalGlucosides from Passifloraalata. J BrazChemSoc 12: 32-36, 2001. |
| [18] | Reginatto FH, Gosmann G, Schripsema J and Schen - kel EP. HPLC/UV Assay of quadranguloside, the major saponin from Passifloraalata leaves. Phytochemical Analysis 15: 195-197, 2004. |
| [19] | Cruz I. A lagarta do cartucho do milho. Circular técnica 21, EMBRAPA/CNPMS. (EMBRAPA/CNPMS, Sete lagoas, pp 1-45 (1995). |
| [20] | SOSBAI. Arroz Irrigado. Recomendações técnicas da pesquisa para o sul do Brasil. SOSBAI/UFSM/IRGA/EPAGRI, Santa Maria, RS 159p., 2005. |
| [21] | Busato GR, Garcia MS, Loeck AE, Zart M, Nunes AM, Bernardi O and Andersson FS. Adequação de uma dieta artificial para os biótopos do “milho” e “arroz” de Spodopterafrugiperda (Lepidoptera: Noctuidae). Bragantia 65: 317-323 (2006). |
| [22] | Oliveira MSS, Roel AR, Arruda EJ and Marques AS. Eficiência de produtos vegetais no controle da lagarta-do-cartucho-do-milho Spodopterafrugiperda (J. E. Smith) (Lepidoptera: Noctuidae). Ciência e Agrotecnologia 31: 326-331, 2007. |
| [23] | Adel MM, Sehnal F and Jurzysta M. Effects of alfalfa saponins on the moth Spodopteralittoralis. J ChemEcol 26: 1065-1078, 2000. |
| [24] | Ikbal C, Monia BHK, Mounir T, Wassila H, Najet R, Dorsaf BA, Mejda D andHabib BHM. Toxicity investigation of Cestrum parquisaponins to Culexpipiens larvae. J BiolSci 4:113-120, 2007. |
| [25] | Ikbal C, Mounir T, Mounia BHK and Habib BHM. Histological Effects of Cestrum parquiSaponins on Schistocercagregaria and Spodopteralittoralis. J BiolSci 7: 95-101, 2007. |
| [26] | Zapata N, Budia F, Vinuela E and Medina P. Antifeedant and growth inhibitory effects of extracts and drimanes of Drimyswinteri stem bark against Spodopteralittoralis (LepNoctuidae). Ind Crop Prod 30: 119-125, 2009. |
| [27] | Pavela R. Antifeedant activity of plant extracts on Leptinotarsadecemlineata Say. andSpodopteralittoralis Bois Larvae. Ind Crop Prod 32: 213-219, 2010. |
| [28] | Pouitout S and Bues R. Élevage de plusieursespèces de LépidoptèresNoctuidaesurmitieuartificiel riche etsur milieu simplifié. Ann EcolAnim 2: 79-91, 1970. |
| [29] | Gulley DD, Boelter AM and Bergman HL. TOXTAT 3.3 Computer Program, 1994. |
| [30] | Callegari-Jacques SM. Bioestatística: princípios e aplicações. Artmed 255p., 2003. |
| [31] | Gazzoni DL, Hülsmeyer A and Hoffmann-Campo CB. Efeito de diferentes doses de rutina e quercetina na biologia de AnticarsiagemmatalisHübner, 1818 (Lep.,Noctuidae). PesqAgropec Bras 32: 673-681, 1997. |
| [32] | Costa ELN, da Silva RFP and Fiuza L.M. Efeitos, aplicações e limitações de extratos de plantas inseticidas. ActaBiologicaLeopoldensia 26: 173-185, 2004. |
| [33] | Augustin JM, Kuzina V, Andersen SB and Bak S. Molecular activities, biosynthesis and evolution of triterpenoidsaponins. Phytochemistry 72: 435-457, 2011. |
| [34] | Rosenthal GA and Berenbaum MR. Herbivores – their interactions with secundary plant metabolites. Vol 1 Academic Press, New York 468p, 1991. |
| [35] | Gershenzon J and Croteau R. Terpenoids. pp.165-219. In: Rosenthal, G.A. &Berenbaum, M.R. (eds.) Herbivores: their interactions with secondary plant metabolites. Academic Press, New York, 468p, 1991. |
| [36] | Sylwia G, Bogumil L. and Wieslaw O. Effect of low and high- saponin lines of alfafa on pea aphid. J Insect Physiol 52: 737-743, 2006. |
| [37] | Goławska S. Deterrence and Toxicity of Plant Saponins for the Pea Aphid AcyrthosiphonPisum Harris. J ChemEcol 33:1598-1606, 2007. |
| [38] | Pelah D. Abramovich Z, Markus A and Wiesman Z. The use of commercial saponin from, Quillajasaponaria bark as a natural larvicidal agent against Aedesaegypti and Culexpipiens. J Ethnopharmacol 81: 407-409, 2002. |
| [39] | Santiago GMP, Viana FA, Pessoa ODL, Santos RP, Pouliquen YBM, Arriaga AMC, Andrade-Neto M and Braz-Filho R. Avaliação da atividade larvicida de saponinas triterpênicas isoladas de Pentaclethramacroloba (Willd.) Kuntze (Fabaceae) e CordiapiauhiensisFresen (Boraginaceae) sobre Aedes aegypti. Rev BrasilFarmacogno 15: 187-190, 2005. |
| [40] | Kumar MS and Maneemegalai S. Evaluation of Larvicidal Effect of Lantana camara Linn Against Mosquito Species Aedesaegypti and Culexquinquefasciatus. Adv Bio Res 2: 39-43, 2008. |
| [41] | Bagavan A, Rahuman AA, Kamaraj C and Kannappan G. Larvicidal activity of saponin from Achyranthesaspera against Aedesaegypti and Culexquinquefasciatus (Diptera: Culicidae). Parasitol Res 103:223-229, 2008. |
| [42] | Waligóra D. Activity of the saponin extract from the bark of Quillajasaponaria Molina, against colorado potato beetle (Leptinotarsadecemlineata Say). J Plant Protec Res 46:199-206, 2006. |
| [43] | Nielsen JK, Nagao T, Okabe H and Shinoda T. Resistance in the Plant, Barbarea vulgaris, and Counter-Adaptations in Flea Beetles Mediated by Saponins. J ChemEcol 36: 277-285, 2010. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML
