-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2012; 2(5): 138-145
doi: 10.5923/j.plant.20120205.01
Heritability and Genetic Advance for Grain Yield and its Component Characters in Maize (Zea Mays L.)
Bello O. B. 1, Ige S. A. 2, Azeez M. A. 2, Afolabi M. S. 2, Abdulmaliq S. Y. 2, Mahamood J. 2
1Department of Biological Sciences, Fountain University, Osogbo, Osun State, Nigeria
21Department of Biological Sciences, Fountain University, Osogbo, Osun State, Nigeria
Correspondence to: Bello O. B. , Department of Biological Sciences, Fountain University, Osogbo, Osun State, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Knowledge of the magnitude of genetic variability, heritability and genetic gains in selection of desirable characters could assist the plant breeder in ascertaining criteria to be used for the breeding programmes. Ten open pollinated maize varieties were evaluated at the Teaching and Research farm, University of Ilorin, Nigeria, during 2005 and 2006 cropping seasons to estimate genetic variability, heritability and genetic advance of grain yield and its component characters. The effect of genotype and genotype by year interaction were significant for ear weight and grain yield, while the effect of year was highly significant (P< 0.01) for all the characters. High magnitude of phenotypic and genotypic coefficient of variations as well as high heritability along with high genetic advance recorded for grain yield, number of grains ear-1, ear weight, plant and ear heights provides evidence that these parameters were under the control of additive gene effects and effective selection could be possible for improvement for these characters. Tze Comp3 C2, Acr 94 Tze Comp5, Tze Comp 4-Dmr Srbc2 and Acr 90 Pool 16-Dt were identified as outstanding genotypes for maize grain yield and should be tested at multilocation for their yield performance.
Keywords: Maize, Heritability, Genetic Gain, Grain Yield, Open Pollinated Maize Varieties
Article Outline
1. Introduction
- Maize (Zea mays L.) has high potential for production and productivity in the savanna ecology of sub-Saharan Africa due to high solar radiation and low night temperatures[1,2]. As a result of its commercial importance in the Nigerian economy, maize is complementing the traditional crops such as guinea corn[Sorghum bicolor (L.) Moench] and millet[Pennisetum glaucum (L.) R. Br.] in parts of the Sudan, Sahel and Guinea ecologies[3]. Maize production has progressively increased over the years with estimated national outputs reaching 7.68 million tonnes in 1995[4]. The projected production and demand by the year 2010 is 13.4 million tonnes[5]. In view of the importance of maize in Nigeria, researchers are utilizing available genetic resources to reconstruct the ideotype of the plant in order to meet the ever increasing requirements of the population through improvement in grain yield, other desirable agronomic and phenological characters as well as quality[6].The success of any crop improvement programme not only dependent on the amount of genetic variability present in the population but also on the extent to which it is heritable, which sets the limit of progress that can be achieved through selection[7-12]. Genetic variability for agronomic characters therefore is a key component of breeding programmes for broadening the gene pool of crops[13]. Heritability is a measure of the phenotypic variance attributable to genetic causes and has predictive function in plant breeding. It provides information on the extent to which a particular morphogenetic character can be transmitted to successive generations. Knowledge of heritability influences the choice of selection procedures used by the plant breeder to decide which selection methods would be most useful to improve the character, to predict gain from selection and to determine the relative importance of genetic effects[14-16]. The most important function of heritability in genetic studies of quantitative characters is its predictive role to indicate the reliability of phenotypic value as a guide to breeding value[17]. Characters with high heritability can easily be fixed with simple selection resulting in quick progress. However, it has been accentuated that heritability alone has no practical importance without genetic advance[8]. Genetic advance shows the degree of gain obtained in a character under a particular selection pressure. High genetic advance coupled with high heritability estimates offers the most suitable condition for selection. Reference[18] reported the limitation of estimating heritability in narrow sense, as it included both additive and epistatic gene effects, and thereby suggested that heritability estimates in the broad sense will be reliable if accompanied by a high genetic advancement. Different researchers[19-22] have reported high heritability and high genetic advance for different yield controlling traits in maize. Therefore, availability of good knowledge of these genetic parameters existing in different yield contributing characters and the relative proportion of this genetic information in various quantitative traits is a pre-requisite for effective crop improvement. The present study was conducted to assess genetic variability, heritability and genetic advance for grain yield and its component characters in ten open pollinated maize varieties to provide necessary information that could be useful in maize improvement programmes aimed at improving grain yield.
2. Materials and Methods
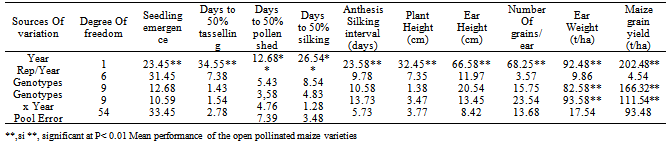
- Ten open pollinated maize varieties (OPVs) were evaluated at the Teaching and Research (T and R) farm of University of Ilorin (Latitude 8°29’N, Longitude 4°35’E) with an annual average rainfall of 945 mm, located in the southern Guinea savanna ecological zone of Nigeria. The OPVs were selected for grain yield and adaptation to abiotic (drought) and biotic (Stalk rot, Striga and Downy mildew) stress factors. They were early to medium maturing white cultivars with maturity period of 90 to 100 days. The cultivars were obtained from the International Institute of Tropical Agriculture (IITA), Ibadan. The origin, genetic background, breeding emphasis and ecological adaptation of the maize parents are presented in Table 1.Soil samples collected from the trial site before cropping in 2005 and 2006 were analyzed in the Soil Science Laboratory of the University of Ilorin, Ilorin, Nigeria for selected physical and chemical properties and presented in Table 2. The collected samples were air-dried and passed through 2mm sieve to remove large particles, debris and stones. The sieved samples were analyzed for pH in 1:1 soil to water ratio using the Coleman pH meter. Organic carbon was determined by Walkley and Black procedure[23]. Total Nitrogen was determined by the micro Kjeldahl method[24], while available phosphorus was extracted by Bray’s P1 method[25] and read from the atomic absorption spectrometer. Exchangeable Ca, Mg, K, Na and effective cation exchangeable capacity (ECEC) were analyzed using Atomic Absorption Spectrophotometery[26], while textural analysis was done by hydrometer method. The soil texture was loamy and the other soil properties were not significantly different in both years at 0-15cm and 15-30cm depth. At 0-15cm depth, the approximate amounts of silt, sand and clay were 8%, 84% and 8%, respectively, with soil pH = 7.30 and ECEC = 2.83 (Cmol kg-1). However, rainfall distribution data for the year 2005 and 2006 were also collected (Fig. 1).
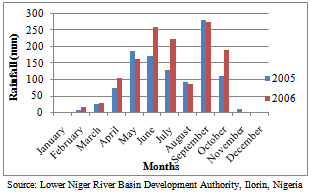 | Figure 1. Monthly rainfall distribution pattern for Ilorin in 2005 and 2006 |
2.1. Statistical Analysis
- Combined analysis of variance and means over years were computed using PROC GLM model of SAS[27] for the OPVs with respect to grain yield and other agronomic characters The mean values were compared using least significant difference (LSD) procedure as laid down according to[28].The linear statistical model was used as described by[29] asYijk = µ + βj + ʎk + (GE)ij + ԐijkWhere: Yijk = the observation made in the ith genotypes on the jth replication, in the kth year; µ = the overall mean of the character; βj = the effect of the jth replication; ʎk = the effect of the kth year; (GE)ij = sum of interaction terms of the genotypes and year, and Ԑijk = the residual effects.The form of the analysis of variance indicating sources of variation, mean squares and their expected values are shown in Table 3. Components of variance were estimated using the method described by[29]. The form of the estimation of the variance components obtained by equating the mean square for a source of variation to its expectation and solving for the unknown is presented in Table 4.Where: δ2g, δ2gy and δ2e are components of genotype, genotype by environment interaction and variance for error respectively. M1, M2, and M3 are the observed values of the mean squares for the error, interaction and genotype respectively[30].
|
|
|
|
|
|
|
|
3. Results and Discussion
3.1. Analysis of Variance
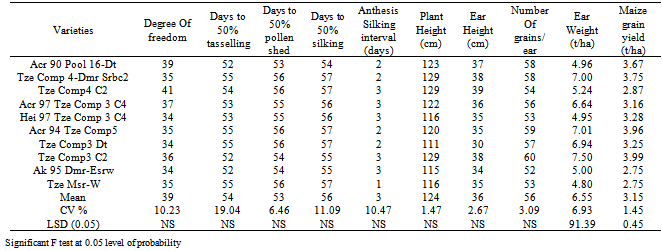
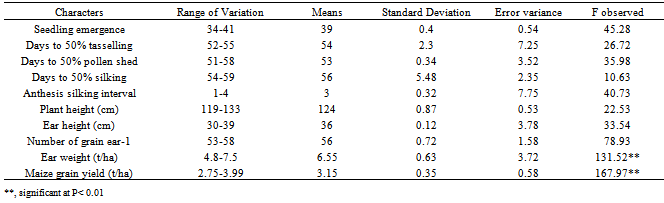
- Combined analysis of variance indicated that the effect of year was highly significant (P< 0.01) for all the characters (Table 5). Rainfall amount and distribution that was higher and favourable in the year 2006 compared to 2005 might have contributed significantly to the differences observed between the years for these traits as differences in environmental conditions varied from year to year. (Fig. 1). Mean squares due to genotypes and genotype x year interaction were significant for ear weight and grain yield indicating presence of genetic variability for these two traits in the germplasm material studied and they were highly infuenced by environmental factors. This also indicated that there was significant amount of phenotypic variability, and that all the genotypes differed from one another with regard to ear weight and grain yield offers way for further improvement through simple selection. However, the interaction of the year with genotypes is very important in this study. The effect of genotype by year interaction was highly significant for ear weight and grain yield. This indicates the diversity of the genotypes and their differences in environmental responses across the two years for these traits. This invariably suggests that maize grain yield could be genetically manipulated for its improvement.The mean performances across the two years for grain yield and related characters of the OPVs is presented in Table 6. The results showed significant differences among the genotypes for growth, yield and yield components. The most outstanding genotypes for grain yield are Tze Comp3 C2, Acr 94 Tze Comp5, Tze Comp 4-Dmr Srbc2 and Acr 90 Pool 16-Dt in descending order with yield ranging from 3.67-3.99 t/ha, while Ak 95 Dmr-Esrw had the lowest value of grain yield (2.75 t/ha) over the two years. Seedling emergence varied from 34-41 with a mean of 39 (Tables 5 and 6) and Tze Comp4 C2 having the highest. The range observed for days to anthesis was 52-55 days with overall mean of 54 days. Acr 90 Pool 16-Dt, Tze Comp3 C2 and Ak 95 Dmr- were the earliest to tassel. Days to silking varied from 54-59 with overall mean of 56 days. Half of the number of genotypes under study took the longest time to silk by silking at 57 days after sowing. The mean plant and ear heights of the genotypes ranged from 119-133cm and 30-39cm respectively. Highest number of grains ear-1 (60) was recorded for Tze Comp3 C2, while Tze Msr-W had the lowest ear weight (4.8 t/ha). The error of variance of the mean for all the traits was small. This might be as a result of optimum number of replications (four) and data from two growing seasons used in estimating the components of variance for the traits. The wide variability observed for grain yield as a quantitatively inherent character among the genotypes means that there is ample opportunity for selection in the genotypes for improvement of this important economic character. This variability could be heritable and exploited in the process of selection in the breeding programmes. However, non-significant F observed values were recorded for the agronomic traits except ear weight and grain yield (Table 7).
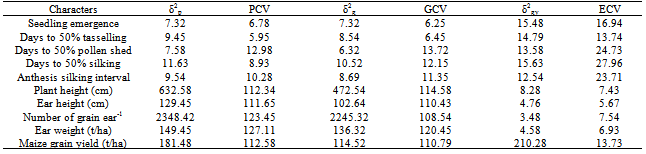
3.2. Phenotypic and Genotypic Coefficients of Variation
- Since most of the economic characters (grain yield) are complex in inheritance and are greatly influenced by several genes interacting with various environmental conditions, the study of phenotypic coefficient of variation (PCV) and genotypic coefficient of variation (GCV) is not only useful for comparing the relative amount of phenotypic and genotypic variations among different traits but also very useful to estimate the scope for improvement by selection. The reliability of a parameter to be selected for breeding programme among other factors is dependent on the magnitude of its coefficient of variations (CV) especially the GCV. However, the differences between genotypic and phenotypic coefficient of variability indicate the environmental influence. While a lower value of CV generally depicts low variability among the tested sample; a high proportion GCV to the PCV is desirable in breeding works. The results given in Table 8 depicted that phenotypic variances (σ2p) and PCVs were slightly higher than genetic variances (σ2g) and GCVs for all the characters, suggesting the least influence of environment in the expression of these characters Similar results have also been reported by[22]. Also, estimates of genotype x year interaction variance (σ 2gy) for the traits in most instances were low. This result tends to support the notion that greater heterozygousity confers a buffering effect or stability over a wide range of environments, whereas inbreeding leads to increased homozygousity of the OPVs and less buffering capacity[34]. High genetic variability for grain yield in the genotypes over years recorded in the test materials suggested that it could be further exploited through improvement and selection programmes[8,9,35,36]. High values of PCV and GCV observed in grain yield followed by number of grain ear -1, ear weight, plant height and ear height not only show that the selection can be effective for these traits but also indicated the existence of substantial variability, ensuring ample scope for their improvement through selection. These observations are in confirmation with the findings of[19,21,22]. On the other hand, very low values of PCV and GCV recorded for seedling emergence, anthesis silking interval days to anthesis, days to pollen shed and silking revealed that low variability among the genotypes was very low for these characters.
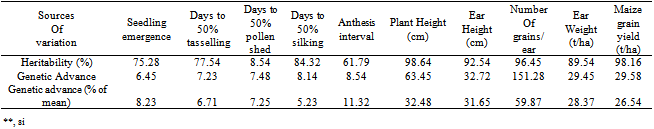
3.3. Heritability and Genetic Advance
- High magnitude of broad sense heritability estimated in all the characters except days to pollen shed (Table 9). This implied the possibility of effective selection for genetic improvement of these traits. High heritability estimates for maize grain yield[9,19,22,35], days to silking and plant height[37] and number of grains ear-1[8,9,20,38,39], observed in the present study were in agreement with the findings of earlier workers. Values of genetic advance as percentage of mean ranged from 59.87 for number of grains ear-1 to 5.23 for days to silk extrusion. High heritability estimates coupled with high estimates of genetic advance expected in the next generation in grain yield, number of grains ear-1, ear weight, plant and ear heights indicate the preponderance of additive gene action for the expression of these traits which is fixable in subsequent generations. This also provides the evidence that larger proportion of phenotypic variance has been attributed to genotypic variance, and reliable selection could be made for these traits on the basis of phenotypic expression. These results find support from the earlier studies by[9,20] that there was greater magnitude of broad sense heritability and high genetic advance in grain yield plant-1, plant height, days to anthesis and silking. The authors suggested that these parameters were under the control of additive genetic effects. Reference[7] also suggested that these parameters could be manipulated according to requirements, and worthwhile improvement could be achieved through selection. Reference[7] concluded that the selection at an early segregating generation will prove beneficial for selecting superior varieties of maize. However, high heritability and low genetic advance were observed for seedling emergence, days to anthesis and silking which may be attributed to non-additive gene action governing these traits, and these characters could be improved through the use of hybridization and hybrid vigour. High heritability accompanied with low genetic advance as per cent of mean in days to tasselling and silking had earlier been reported by[7]. Days to pollen shed exhibited low heritability with low genetic advance indicating non-additive genetic effects governing this trait. Moderate heritability along with high genetic advance was recorded for anthesis-silking interval providing little chance for its further improvement. However, care must be taken while breeding for this complex trait as it is considerably influenced by environmental factors. It seems a limited scope of improvement could be achieved for this trait within this group of genotypes. Maize grain yield, number of grains ear-1, ear weight as well as plant and ear height can be improved by selection, as these characters exhibited moderate genotypic and phenotypic coefficient of variations along with both medium to high heritability and genetic advance. Seedling emergence, anthesis-silking interval, days to anthesis and silking had high heritability but the genetic coefficient of variations was low. This indicates that though, the character is highly heritable, its improvement through early generation selection may not give the desired results. Low genetic coefficient of variation and heritability obtained for days to pollen shed is not particularly surprising since yield is a product of many complex characters. Therefore, direct selection for days to pollen shed improvement may not be possible, but through indirect selection of other secondary traits may be feasible.
4. Conclusions
- This study revealed that information about the extent of variation, estimates of heritability and expected genetic advance in respect of maize grain yield and yield contributing characters constitutes the basic requirement for a crop improvement programme. Broad sense heritability is useful for measuring the relative importance of additive portion of genetic variance that can be transmitted to the offspring. The preponderance of additive gene effects controlling a trait usually resulted to both high heritability and genetic advance, while those governed by non-additive gene actions could give high heritability with low genetic advance. However, in the present research, expected genetic advance values were based on broad sense heritability, which integrates additive portion of the total phenotypic variance. Effective selection for superior genotypes is possible considering grain yield, number of grains ear-1, ear weight, plant and ear heights and could be used as target traits to improve maize grain yield. The most outstanding genotypes (Tze Comp3 C2, Acr 94 Tze Comp5, Tze Comp 4-Dmr Srbc2 and Acr 90 Pool 16-Dt) for grain yield could be sources of alleles that can be manipulated with other promising cultivars for higher grain yield in this southern Guinea savanna ecology.
References
| [1] | Kassam AH, Kowal J, Dagg M, and Harrison MN (1975). Maize in West Africa and its potential in the savanna area. World Crops, 27: 73-78. |
| [2] | Undie UL, Uwah DF and Attoe EE (2012). Growth and development of late season maize/soybean intercropping in response to nitrogen and crop arrangement in the forest agro-ecology of south southern Nigeria. Int. J. Agric.Res., 7(1): 1-16. |
| [3] | Kim SK (1991). Breeding maize for Striga tolerance and the development of a field infestation technique. In Kim SK (ed.) Combating Striga in Africa: Proc. Int. Workshop by IITA ICRI-SAT and IDRC, Ibadan, Nigeria. 22-24 August, 1988, IITA, Ibadan, pp 96-108. |
| [4] | Edache OA (1996). Maize production and utilization in Nigeria: Problems and prospects. In: Proceedings of National Workshop on Maize Production, Kaduna, Nigeria, pp 3-8. |
| [5] | Federal Ministry of Agriculture and Natural Resources (1995). FMANR, Nigeria. National Agricultural Research Strategic Plan (1996-2010), 1: 140-142. |
| [6] | Bello OB, Abdulmaliq SY, Afolabi MS, and Ige SA (2010). Correlation and Path coefficient analysis of yield and agronomic characters among open pollinated maize varieties and their F1 hybrids in a diallel cross. Afri. J. Biotech., 9(18): 2633-2639. |
| [7] | Sumathi P, Nirmalakumari A and Mohanraj K (2005). Genetic variability and traits interrelationship studies in industrially utilized oil rich CIMMYT lines of maize (Zea mays L). Madras Agric. J. 92 (10-12): 612-617. |
| [8] | Najeeb S, Rather AG, Parray GA, Sheikh FA and Razvi SM (2009). Studies on genetic variability, genotypic correlation and path coefficient analysis in maize under high altitude temperate ecology of Kashmir. Maize Genetics Cooperation Newsletter. 83: 1-8. |
| [9] | Kashiani P, Saleh G, Abdullah SN and Abdullah NAP (2008). Performance, heritability correlation studies on nine advance sweet corn inbred lines. Proceeding of the 10th Symposium of Malaysian Society of Applied Biology, Nov. 6-8, 2008, Malaysia, pp 48-49. |
| [10] | Hussain N, Khan MY and Baloch MS (2011). Screening of maize varieties for grain yield at Dera Ismail Khan. J. Animal and Plant Sci., 21(3): 626-628. |
| [11] | Khan K, Sher H, Iqbal M and Al-Qurainy F (2011). Development and release of indigenous maize hybrids to enhance maize yield in Khyber-Pakhtoonkhua province of Pakistan. Afri. J. Agric. Res., 6(16): 3789-3792. |
| [12] | Wang X, Chang J, Qin G, Zhang S, Cheng X and Li C (2011). Analysis on yield components of elite maize variety Xundan 20 with super high yield potential. Afr. J. Agric. Res., 6(24): 5490-5495. |
| [13] | Ahmad SQ, Khan S, Ghaffar M and Ahmad F (2011). Genetic diversity analysis for yield and other parameters in maize (Zea mays L.) genotypes. Asian J. Agric. Sci., 3(5): 385-388. |
| [14] | Waqar-Ul-Haq M, Malik F, Rashid M, Munir M and Akram Z (2008). Evaluation and estimation of heritability andgenetic advancement for yield related attributes in wheat lines. Pak. J. Bot., 40(4): 1699-1702. |
| [15] | Kashiani P, Saleh G, Abdullah NAP and Abdullah SN, (2010). Variation and genetic studies on selected sweet corn inbred lines. Asian J. Crop Sci., 2(2); 78-84. |
| [16] | Laghari KA, Sial MA, Afzal Arain MA, Mirbahar AA, Pirzada AJ, Dahot MU and Mangrio SM (2010). Heritability studies ofyield and yield associated traits in bread wheat. Pak. J. Bot., 42(1): 111-115. |
| [17] | Falconer DS and Mackay TFC (1996). Introduction to quantitative genetics. 4th ed. Benjamin Cummings, England, pp. 245-247. |
| [18] | Ramanujam S and Thirumalachar DK (1967). Genetic variability of certain characters in red pepper (Capsicum annum). Mysore J. Agric. Sci., 1: 30-36. |
| [19] | Rafique M, Hussain A, Mahmood T, Alvi AW and Alvi B (2004). Heritability and interrelationships among grain yield and yield components in maize (Zea mays L). Int. J. Agri. Biol., 6(6): 1113-1114. |
| [20] | Akbar M, Shakoor MS, Hussain A and Sarwar M (2008). Evaluation of maize 3-way crosses through genetic variability, broad sense heritability, characters association and path analysis. J. Agric. Res., 46(1): 39-45. |
| [21] | Rafiq CM, Rafique M, Hussain A and Altaf M (2010). Studies on heritability, correlation and path analysis in maize (Zea mays L.). Agric. Res., 48(1): 35-38. |
| [22] | Nelson DW and Somers LE (1992). Total carbon, total organic carbon and organic matter. In: Miller et al. (ed). Methods of soil analysis, Part 2, 2nd ed. Agronomy Monograph, 27, ASA, Madison, W. I., pp. 539-580. |
| [23] | Bremner JM (1965). Total nitrogen. I methods of soil analysis. II chemical and microbiological properties. In: Black CA, Evans DD, White JL, Ensminger LE, Clerk FE and Dinauer RC (eds): Agronomy Monograph, 9, American Society of Agronomy Madison, Wisconsin. U.S.A. |
| [24] | Bray RH and Kurtz LT (1945). Determination of total organic and available forms of phosphorus in soils. Soil Sci., 59: 39-45. |
| [25] | International Institute of Tropical Agriculture (1989). Automated and semi-automated methods for soil and plant analysis. Manual series No. 7, IITA, Ibadan, Nigeria. |
| [26] | SAS Institute, (2007). SAS system for windows version 9.2. SAS Institute. Cary, NC. |
| [27] | Steel RGD, Torrie JH and Dickey DA (1980). Principles and procedures of Statistics: A biometrical approach. 3rd ed, McGraw Hill. Book Co. Inc. New York. USA. |
| [28] | Bliss FA, Baker LN, Franckowiak JD and Half TC (1973). Genetic and environmental variation of seed yield, yield components and seed protein quality of cowpea. Crop Sci., 13: 656-660. |
| [29] | Fehr WR (1987). Principles of Cultivar Development. Macmillan publishing company. A division of Macmillan Inc.New York, 1: 1-465. |
| [30] | Eckebil JP, Ross WM, Gardner CO and Maranville JW (1977). Heritability estimates, genetic correlations and predicted gain from S1 progeny tests in three grain sorghum random mating population. Crop Sci., 17: 363–377. |
| [31] | Allard RW (1960). Principles of plant breeding. 1st Edn., John Wiley and Sons Inc., New York, pp 213-218. |
| [32] | Burton GW (1952). Quantitative inheritance in grasses. 6th International Grassland Congress. 1: 277-283. |
| [33] | Ajala SO, Ago C and Olaoye G (2009). Comparison of predicted responses to three types of recurrent selection procedures for the improvement of a maize (Zea mays L.) population. J. Plant Breed. Crop Sci., 1(8): 284-292. |
| [34] | Nigussie M and Saleh (2007). Genetic variability and heritability within sweetcorn (Zea mays saccharata) breeding population. Malays. Appl. Biol., 36; 5-20. |
| [35] | Wannows AA, Azzam HK and Al-Ahmad SA (2010). Genetic variances, heritability, correlation and path coefficient analysis in yellow maize crosses (Zea mays L.). Agric. Biol. J. N. Am., 1(4): 630-637. |
| [36] | Aziz K, Rehman A and Rauf A (1998). Heritability and interrelationships for some plant traits in maize single crosses. Pak. J. Biol. Sci., 1(4): 313-314. |
| [37] | Mahmood Z, Malik SR, Akhtar R and Rafique T (2004). Heritability and genetic advance estimates from maize genotypes in Shishi Lusht, a valley of Krakurm. Int. J. Agric. Biol., 6(5): 790-791. |
| [38] | Ojo DK, Omikunle OA, Oduwoye OA, Ajala MO and Ogunbayo SA (2006). Heritability, character correlation and path coefficient analysis among six inbred lines of maize. World J. Agric. Sci., 2 (3): 352-358. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML