-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2012; 2(4): 103-107
doi: 10.5923/j.plant.20120204.01
In Vitro Mutation Induction and Selection of Chrysanthemum (Dendranthema Grandiflora Tzelev) Lines with Improved Resistance to Septoria Obesa Syd
B. Kumar , S. Kumar , M. Thakur
Department of Biotechnology, University of Horticulture & Forestry, Solan, Himachal Pradesh,173230, India
Correspondence to: S. Kumar , Department of Biotechnology, University of Horticulture & Forestry, Solan, Himachal Pradesh,173230, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This paper describes in vitro mutation selection technique for improved resistance in chrysanthemum (Dendranthema grandiflora Tzelev) cv. Snow Ball against culture filtrate of Septoria obesa, a leaf spot pathogen. The callus was initiated from leaf explant on MS medium supplemented with 10 mg/l kinetin and 1 mg/l NAA. Optimal doses of gamma radiation and culture filtrate on the per cent survival of calli were standardized. The optimal dose of gamma radiation was 20 Gy and that of culture filtrate was 15%. The selection of the resistant calli was made at 15% culture filtrate concentration and the calli were subjected to two more cycles of selection (30 day cycle) to obtain resistant cell lines. 100% of the plants raised from cuttings had acquired resistance against the pathogen in the pot in greenhouse. The plants were maintained for four years without any symptoms with regular inoculation of fungal spore suspension. No phenotypic variation was observed in the resistant plants.
Keywords: Culture filtrate, Growth regulators, In vivo testing, Leaf spot pathogen, Resistance
Article Outline
1. Introduction
- Chrysanthemum is one of the most important commercial cut flowers of the world and is the second largest in demand after rose, among the ornamental plants traded in the global flower market1. Economic returns from cut flowers mainly depend upon the quality of the flowers2. Garden chrysanthemums are the number one herbaceous perennial in United States with whole sale farmgate value of $141.8 million in 20053. Diseases caused by fungi, bacteria, mycoplasma, and viruses are responsible for poor quality of flowers and reduction in yield4.Septoria obesa Syd is one of the most important pathogens of chrysanthemum. This pathogen causes leaf spots, resulting in 15-20% yield loss. The control of leaf spot pathogen by fungicides is difficult because fungicides are often ineffective as the pathogen spreads rapidly under favourable conditions. On the other hand, major destructive fungi are developing resistance to most classes of fungicides and environmental pollution caused by these chemicals is a serious problem5,6. Genetic resistance in D. grandiflora is therefore of considerable importance. Mutation techniques have been widely applied to improve crop yield, quality, disease and pest resistance7. About 2335 varieties were released through mutagenesis in the world, in which ornamental crops and decorative crops are 5528. In chrysanthemum mutant cultivars can account for almost 40% of the Dutch flower market and about 236 officially announced commercial mutants have been obtained through X- or γ–ray mutation breeding9. A large number of germplasm with novel, desired traits have been produced through mutation techniques10-13. Combining mutation technique with in vitro selection, allow the selection of traits controlled by their recessive or dominant genes, can improve recovery of the desired mutant. In vitro mutation and selection has advantages over traditional breeding methods as these are more efficient and cost effective. In order to screen target characters in a large number of mutagenised cells, it is essential to have efficient selection agents7. Toxic culture filtrate and purified toxins have been used in in vitro selection of disease resistant plants14-16. It has been attempted at the plant level where a large population of plants, raised through in vitro callus cultures, have been screened for resistance in the field capacity or via a more targeted approach of regeneration of disease resistant plants through resistant callus culture selected against fungal toxins17. Kumar et al.16 reported resistant cell lines of chrysanthemum against Septoria obesa. In this paper the development chrysanthemum cv. Snow Ball with an improved resistance to Septoria obesa, is described.
2. Materials and Methods
2.1. Plant Materials
- Chrysanthemum (Dendranthema grandiflora Tzelev) cv. Snow Ball, growing in the Department of Biotechnology, University of Horticulture and Forestry, Solan, Himachal Pradesh, India was propagated from cuttings and maintained under greenhouse at 24±2℃ with 80% relative humidity.. Young leaves (upper 3-4 leaves)isolated from the one-year- old plants washed thoroughly under running water, cut into small segments (0.2-0.5 cm) and were used as explants. The explants were surface sterilized with 5% sodium hypochlorite and washed 3-4 times with sterile water prior to culturing. The callus cultures were initiated by culturing the leaf explants on MS18 medium containing 3% sucrose (w/v), 10 mg/l kinetin and 1 mg/l NAA. The pH of the medium was adjusted to 5.8 before autoclaving. The medium was solidified with 0.8% (w/v) agar and autoclaved at 121℃ for 15 min. Erlenmeyer conical flasks (100 ml) were cast with 30 ml media and sealed with cotton plugs made from non-absorbent cotton. The cultures were incubated under cool fluorescent lamps at an light intensity of 50-60 µmol m-2 s-1 with a light/dark cycle of 16/8 h at 24±2℃. All the cultures were transferred to the fresh medium at 30 days interval.
2.2. Determination of Optimal Dose of Gamma Ray
- To determine the suitable dose of gamma ray calli (50 mg fresh weight) were cultured on MS medium and exposed to gamma irradiation of 0, 10, 20 and 30 Gy (cobalt 60). About 50 calli pieces were tested for each dose. The surviving calli were subcultured onto fresh MS medium supplemented with growth regulators used for callus induction and growth. (After calli irradiation, did you allow to grow on the same medium or transferred to the fresh medium for further growth). The calli were observed for their growth after 30 days recovery period, after which surviving calli were counted, weighed and LD50 (half lethal dose) of gamma rays was estimated. (What is the parameter you used for the determination of LD50 dose)IAEA, Vienna has been carrying out work on induced mutations. You have not quoted.
2.3. Maintenance and Multiplication of the Pathogen Cultures
- The pure cultures of S. obesa were obtained from the department of Mycology and Plant Pathology, University of Horticulture and Forestry, Solan (H.P.), India. The isolate was multiplied on PDA medium at 25℃ and maintained at 4℃ on the same medium. The pH of the PDA medium was adjusted to 6.0–6.5. After inoculation, the cultures were incubated at 25℃ in the dark for 21 days until a uniform fluffy mycelial growth was obtained.
2.4. Preparation of Culture Filtrate
- The culture filtrate was prepared by inoculating I mm2 . of the fungal mycelium in 100 ml liquid Czapek dox medium.The culture was incubated in a BOD incubator shaker at 25℃ in dark. After 21 days the fungal cultures were filtered through Whatman filter paper no. 1 and the filtrate centrifuged at 20, 000 rpm for 30 min. The supernatant was then filtered through sintered glass filter (G-5 grade, 0.25 µm pore size) to produce transparent culture filtrate.
2.5. Determination of Optimal Concentration of Culture Filtrate for Screening
- To determine the effects of culture filtrate on survival and growth of calli, one hundred pieces of mutated calli (20 mg fresh weight) per culture filtrate treatment, in ten flasks (100 ml), each having 10 pieces, were inoculated on MS medium containing 0%, 2.5%, 5.0%, 7.5%, 10%, 12.5%, 15%, 17.5% and 20% (v/v) culture filtrate and the growth regulators used for callus induction and the medium was solidified with 0.8% agar. In control cultures an equal volume of Czapek dox medium replaced with culture filtrate. The cultures were maintained at light intensity of 50-60 µmol m-2 s-1 under light/dark cycle of 16/8 h at 24±2℃ for four weeks. The calli obtained from the selection medium containing 15% culture filtrate, the highest concentration of culture filtrate at which only few calli survived, were subcultured on the same medium for two more cycles (30 day cycle) to select resistant calli. The surviving calli were cultured on MS medium supplemented with 10 mg/l kinetin and 1 mg/l NAA for multiplication. The pH of the medium was adjusted to 5.8.
2.6. Plant Regeneration from Calli
- After determining the optimal concentration of culture filtrate, the surviving calli were induced to regenerate plantlets. For shoot regeneration, calli were transferred onto MS medium supplemented with 0.2 mg/l BA, 0.2 mg/l NAA and 1 mg/l GA3 without culture filtrate. After shoot formation, they were separated from the callus mass and cultured on MS medium containing 0.3 mg/l IBA and 0.2% activated charcoal for rooting. The plantlets were maintained at 24±2℃ under 16/8 h (light/dark) photoperiod. The plants with well developed root systems were transferred to pots containing soil:sand (1:1) mixture and grown in the greenhouse. The average number of shoots and roots produced were recorded to examine the effect of culture filtrate concentration on plant regeneration.
2.7. Leaf Spot Resistance of Selected Regenerated Plants
- Hundred plants (one plant in each pot) were tested for resistance against leaf spot pathogen. The plants were subjected to infection by spraying spore suspension (40 spores/ml) of S. obesa and were observed daily for the development of symptoms. Disease severity was recorded according to 0-4 rating scale16. Two hundred cuttings (5-6 cm long) were collected from one-year-old resistant plant and treated for 24 h with 100 mg/l IBA solution for rooting. The rooted cuttings were transferred to pots containing soil: sand (1:1) and maintained in greenhouse for three months before spraying with spore suspension (40 spores/ml) of S. obesa. The plants were observed daily for the appearance of symptoms. The resistant plants were maintained for four years in the greenhouse and inoculated regularly by spraying spore suspension of the pathogen and observed for the development of symptoms.
2.8. Statistical Analysis
- The experiment was repeated three times with similar trends of results using completely randomized design19. The significance of treatment effects on various parameters was determined using analysis of variance (ANOVA).
3. Results and Discussion
- To determine the optimal dose of gamma rays (optimal dose not concentration), the calli were exposed to 10, 20 and 30 Gy gamma ray doses. The percentage of surviving calli decreased with the increasing gamma ray doses (Figure 1). There was no calli recovered at gamma ray dose 30 Gy, about 22.2% of the calli survived at 20 Gy dose of gamma irradiation. Therefore subsequent physical mutation was carried out only with 20 Gy of gamma irradiation. To determine the effects of culture filtrate, survival of the calli decreased with the increase in the culture filtrate concentration, reaching a 0% survival at 17.5% culture filtrate (Figure 2). A significant negative correlation was found between culture filtrate concentration and average survival of calli. The cell selection was therefore carried out at the 15% culture filtrate level to exert maximum selection pressure that allowed the recovery of resistant lines. The selected calli were subcultured on culture medium in the absence of culture filtrate for three subcultures to get rid of physiological adaptations and again cultured at 15% culture filtrate for two more cycles (30 day cycle) before the plants were regenerated.
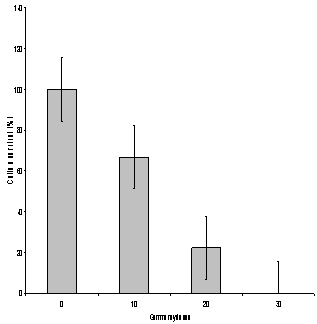 | Figure 1. Effect of gamma ray doses on average survival of calli (%). Vertical bars represent LSD (P≤ 0.05). |
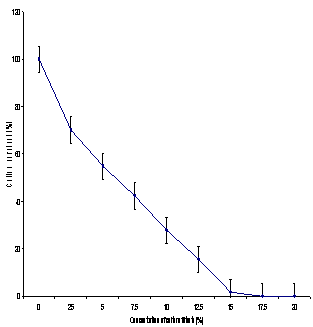 | Figure 2. Effect of different concentrations of culture filtrate on percent survival of calli. Vertical bars represent LSD (P ≤ 0.05) |
 | Figure 3. control (a) and resistant plants (b) of chrysanthemum in pots under glasshouse |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML