-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2012; 2(2): 41-45
doi: 10.5923/j.plant.20120202.08
Comparison of leaf morphoanatomy of Diodella radula (Willd. & Hoffmanns. Ex Roem. & Schult.) Delprete and Diodella teres (Walter) Small (Rubiaceae)
Rosilda Mara Mussury 1, Zefa Valdivina Pereira 1, Silvana De Paula Quintão Sc 2
1Universidade Federal da Grande Dourados/UFGD. Faculdade de Ciências Biológicas e Ambientais. Programa de Pós Graduação em Biologia Geral/Bioprospecção.Rodovia Dourados-Itahum, Km.12,. 79804-970, Dourados-MS-Brasil
2Universidade Federal da Grande Dourados/UFGD. Faculdade de Ciências Agrárias. Programa de Pós Graduação em Agronomia/Produção vegetal. Rodovia Dourados-Itahum, Km.12,. 79804-970, Dourados-MS-Brasil
Correspondence to: Rosilda Mara Mussury , Universidade Federal da Grande Dourados/UFGD. Faculdade de Ciências Biológicas e Ambientais. Programa de Pós Graduação em Biologia Geral/Bioprospecção.Rodovia Dourados-Itahum, Km.12,. 79804-970, Dourados-MS-Brasil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Diodella radula (Willd. & Hoffmanns. ex Roem. & Schult.) Delprete e Diodella teres (Walter) Small (Rubiaceae) are weeds found in agricultural areas from Mato Grosso do Sul and it is difficult to identify them on vegetative phase, so the morphologic and anatomic studies are an important instrument for controling the species. This work intended to characterize the leaf morphoanatomy of Diodella radula and D. teres, aiming to promote taxonomic subsidies and determine the structures which may influence on the herbicides absorption. The morphoanatomic studies were carried out with fresh material and fixed. Cross and paradermic sections of leaf blade were obtained. The cutting edges were dyed as an usual methodology and submitted to histochemical tests. These species are distinguished plants because they can stay in the sun; Diodella teres is frequently found on the edge of the roads, on a drier soil while D. radula is mainly found on dirty fields, damp soil and at the river banks. D. radula and D. teres leaves present general characteristics quoted for Rubiaceae family, as for example, unilayered epidermis, paracytic stomata, dorsiventral mesophyll and collateral vascular bundles. The observed characteristics such as epidermic cells, papilla size, glandular trichomes and collenchyma arrangement are distinct for both species and therefore they may be used as characters to bound the two species. The low density of stomata and the cuticle thickness on the adaxial surface observed in D. radula and D. teres seems to be leaf barrier to block the herbicides penetration.
Keywords: leaf anatomy, Rubiaceae, weed, epidermic papilla
1. Introduction
- Among the weeds found in agricultural areas in Mato Grosso do Sul and which are difficult to be identified during vegetative phase, Diodella radula (Willd. & Hoffmanns. ex Roem. & Schult.) Delprete and Diodella teres (Walter) Small (Rubiaceae) are the prominent species.Morphology and anatomy aspects of these plants were not observed in literature. So, the structural understanding will be an important instrument to point out the most appropriate strategy and also to minimize environmental impacts by using the most efficient, suitable and the least pollutant herbicides as well as to promote subsidies for taxonomic studies of the group since it presents many problems of generic delimitation and infrageneric classification. Fruit morphology is the main characteristic to distinguish it from the other genera of the tribe, which induces many authors to have a false identification of these species.Some morphlogical and anatomic studies have related the ease or the difficulty of herbicides absorption by the leaves. According to[1], the leaves morphology influences on the quantity of the product was interrupted or retained but it is the leaf anatomy that determines the ease they will be absorbed. So, the leaf anatomy study of the weeds is distinguished as an important technique to identify and describe the structures which may influence on the herbicides absorption besides to assist on the differentiation of the species. [1]consider the thick cuticle, the high content of epicuticular wax, the high density of trichomes, the low stomatic density and the high epidermis thickness in the adaxial side as the major barriers to herbicides penetration in weeds leaves.[2] emphasize the morphologic diversity of leaf epidermis in several species, and denote the topography, the trichomes and stomata types influence on the deposition of the herbicides on the leaf surface.[3]denote the leaf anatomic study may improve the understanding of the barriers each species determine to the herbicides penetration and so, promote subsidies to search the control strategies. Thus, the leaf anatomic analysis may contribute with the studies to select herbicides for these species.Therefore, this work intended to characterize the leaf morphoanatomy of Diodella radula and D. teres, which occur in agricultural areas, aiming to promote taxonomic subsidies and determine the structures which may influence on the herbicides absorption.
2. Material and Methods
- Diodella radula and D. teres are herbs that occur in agricultural areas in Mato Grosso do Sul, comprising Naviraí, Jateí and Taquarussu cities. The botanical materials were identified through a specialist consult and the control was included in the collection of UEC herbarium (Universidade Estadual Campinas), under numbers 146858 and 146878, respectively. The morphologic and anatomic studies were carried out with fresh material and then fixed. Ten leaves of different individuals were collected and anatomically analyzed. Fully-expanded leaves from the 4th node were fixed in FAA50 (formaldehyde, acetic acid and ethanol 50% (1:1; 18, v/v)) for 48 hours and stored in ethanol 70%[4]. The obtained cross sections from the medium third part of leaf in the primary rib, midrib and edge regions were clarified with hypochlorite of sodium 20% and after they were washed in acetic water 2%, they were submitted to a dual coloration with Astra blue and Safranine[5] and arranged in glycerinated jelly[6]. For studying the epidermis, on the medium third part of the leaf and edge, some paradermal cuts were made by hand with a steel blade in the median region of the leaf in the adaxial and abaxial surfaces and some prints with an instant tape (Super Bonder®) were also made to analyze stomata and trichomes types and the shape of common epidermal cells. It was achieved the dissociation technique by crushing method from Jeffrey[4] using aqueous safranine for coloring. Ten measures from papilla and cuticle were carried to a cool material by using an ocular micrometric, in the inner rib region and with the obtained data, the mean values were estimated. Stomata and epidermal cells (mm2) countings were carried out with the assistance of a bright camera, projecting the quadrant of the noted area. Ten fields with six leaves were observed, totalizing 60 fields for each species. For calculating the stomatal index of each species, it was used Salisbury’s formula presented by[7]. Stomata classification was made according to[7], vascular system one was made according to the proposed diagram by[8] and the morphological description followed[9] recommendation. The obtained results were registered with photographs of the entire plant, with a Nikon machine. After analyzing the slide, photomicrographs were produced in a binocular microscope with a connected camera and a selecting image program – Moticam 2300 3.0MP live Resolution.
3. Results and Discussion
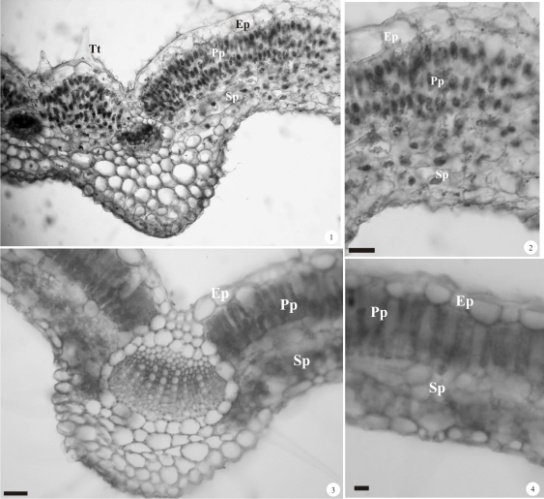
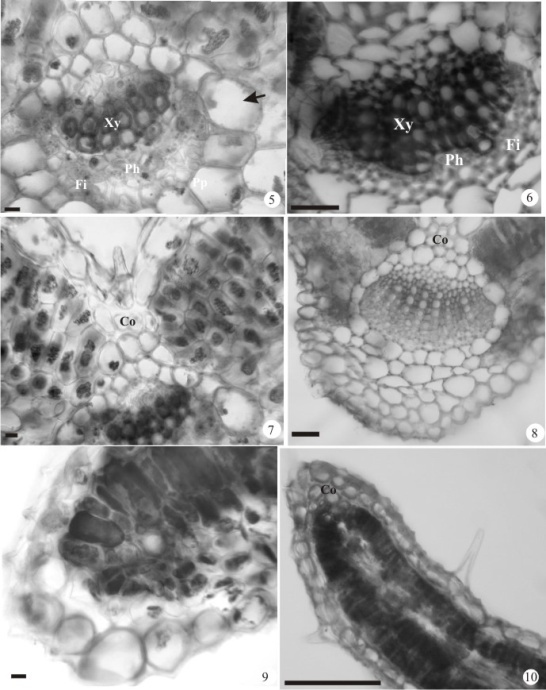
- Diodella radula is distinguished as an erect herb with 40cm height. The leaves are sessile, lanceolated blade and a dark green color, 2-5 x 0.6 - 1cm. The primary and secondary ribs are prominent on the abaxial surface. Five to seven couples of secondary ribs occur. The blade has a sharp tip; attenuated basis; the whole margin slightly revolved; 5-6 fimbriated stipules.Diodella teres is an erected herb with 10 - 40cm height. It has tetragonal and villous branches. The leaves are opposite, sessile, linear-lanceolated blade, green-yellowish color, 1.5 - 4 x 0.5 - 1cm. The main rib is prominent on the abaxial surface and the secondary ones are conspicuous. The blade has an acuminated tip; attenuated basis; whole margin; 6-8 fimbriated stipules.The leaf of the studied species in a paradermal section presents paracytic stomata, the walls of the epidermal cells are slightly frizzy on both surfaces of D. radula and in D. teres, the walls are sinuous on the abaxial surface and straight on the adaxial one. Paracytic stomata, arranged only on the abaxial surface, were described by[10] as the most common type of stoma for Rubiaceae family. Yet, on studied species the stomata were observed on both surfaces of the epidermis. With a front view, epidermal cells on both surfaces present walls slightly frizzy on D. radula and on D. teres, the abaxial side is sinuous and straight on the adaxial one.[11] and[12] remark that the leaf blade, on frontal view, of Melanopsidium nigrum Colla and Palicourea longependuculata (Gardner) Müll. Arg., present anticlinal straight walls on adaxial surface and slightly sinuous one on abaxial side, respectively. For confirming these presented data,[13] observed anticlinal walls on abaxial side with a sinuous wall on Tocoyena bullata (Vell.) Mart., a sunlight plant. However, according to[14] shady plants present sinuous anticlinal walls. But[15] noted epidermal cells with straight wall on shady plants of Psychotria nuda (Cham. & Schltdl.) Wawra and P. leiocarpa Cham. & Schltdl. For[7], on sunny plants of a dry environment, the epidermal cells, specially on adaxial side, present straight wall and on shady plants, the occurrence of cells with sinuous walls on both sides of the leaf is commonly observed. Although, in front of the revealing, it is verified that variation on cells shape is common, but since both species are sunny plants, it is believed that other factors may influence on this characteristic, such as altitude[16], hydric stress[17] and CO2 concentration[18];[19].[20] emphasizes that several researches have demonstrated an intra-specific variation of epidermis characteristics, tending to increase the sinuosity, under conditions with lower solar irradiation.[21], while studying the anatomical differences in Bauhinia radiata Vell. leaves, verified that on shady leaves the anticlinal walls of epidermal cells are sinuous and on sunny leaves, they are straight. According to Table 1, it is observed that the stomata and non-glandular trichomes index is higher on abaxial than on adaxial surface, on both species. In cross section of the midrib, it was observed that the epidermis of both species is unilayered, papillose with a thin and straight cuticle. However, as observed on Table 2, papilla size differs significantly on both species; bigger in D. radula
|
|
ACKNOWLEDGMENTS
- We thank the students Rosmarie de Oliveira Guedes, Paula Sabino Doreto and Patrícia Perez Machado for collaborating during the field work. We also thank the Universidade Federal da Grande Dourados for the financial support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML