-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2012; 2(2): 6-13
doi: 10.5923/j.plant.20120202.02
Growth, Photosynthesis and Yield of Chickpea as Influenced by Urban Wastewater and Different Levels of Phosphorus
Hamid Iqbal Tak 1, Faheem Ahmad 1, O. O Babalola 1, Arif Inam 2
1Department of Biological Sciences, Faculty of Agriculture, Science and Technology, North-West University, Mafikeng Campus, Private Bag X2046, Mmabatho 2735, South Africa
2Environmental and Plant Physiology Lab, Department of Botany, Aligarh Muslim University, Aligarh, 202002, India
Correspondence to: Hamid Iqbal Tak , Department of Biological Sciences, Faculty of Agriculture, Science and Technology, North-West University, Mafikeng Campus, Private Bag X2046, Mmabatho 2735, South Africa.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The use of effluents or marginal water in agriculture is an unavoidable practice and its largest source is the urban wastewater. In order to evaluate its effect on agricultural crops, an experiment was conducted to study its impact on Cicer arietinum L. along with different levels of phophorus. Three levels of water used for irrigation included ground water (GW), 50% wastewater (50%WW) and 100% wastewater (100%WW) and the varying levels of phosphorus (P0, P20, P40, P60) were also applied along with the basal application of nitrogen and potassium at the rate of 15 and 20 kg ha-1 respectively. The effect was evaluated on the basis of growth, biochemical attributes, photosynthesis and yield of the crop. From the results it was clear that the wastewaters proved not only effective source of water but the nutrients also and enhanced growth and photosynthetic capacity and thereby yield of the plants. Moreover the fertilizer (P) requirement of the crop was also reduced. In conclusion the urban wastewater showed no inhibitory effect on the growth of the crop and thus could effectively supplement not only the nutrient requirement of the crop but may also act as the source of water.
Keywords: Wastewater, Phosphorus, Photosynthesis, Yield and Chickpea
Article Outline
1. Introduction
- The problem of water shortage dictates the utilization of marginal water for irrigation and its improper use poses challenges to self sufficiency in food production. Of the current list of 30 or so countries that have per capita runoff of less than 1,700 m3 per year, almost all are net importers of more than 20 percent of their grain requirement. The net import of these water deficient countries already exceeds 25 percent of the global grain trade[1]. If countries like India and China with so much of their population join the water deficit group in couple of decades, the strain on the exporting countries may certainly become unsustainable. Despite growing concern over water shortages and food security, irresponsible overuse and misuse of water continue unabated. Rainfall over land is the main source of fresh water fit for the agriculture and human consumption. Out of the annual rainfall, a good amount of water evaporates back into the atmosphere which is called “Green Water” and is the source of water for all non irrigated vegetation including forests, wood lands and rain fed crops. After allowing for evaporation from continental rainfall nearly 40,000 billion m3 of fresh water enters lakes, reservoirs, streams and aquifers in active exchange with the surface waters. This is called “Blue Water”. It is not only unevenly distributed but also transient as it also evaporates or flows into unusable water sinks such as sea or salt pans. While the remaining surface water is also not wholly accessible, as it flows to remote inaccessible destinations where it is not in demand and sometimes also polluted during its flow as a result of the anthropogenic activities[2,3].India is a developing and industrially fast growing country. It is one of the most densely populated land spaces of the earth. A few monsoon months starting from June to August bring copious rainfall. Its land area of 3.29 million km2 has an annual precipitation of 117 cm. Out of this 4000 billion m3 of rainfall, due to limitation in storing the flood flows of the monsoon rain, only 690 billion m3 of surface water and 450 billion m3 ground water remain fit for use. But due to uneven distribution of water availability over time and space we have typical flood-drought syndrome prevailing in several parts of the country and against these estimates of water availability, we have the demand projection of around 1000 billion m3 with in next few years[4]. According to the Central Pollution Control Board of India (CPCB), the total wastewater generated by industry in 2004-05 was 26,254 ML/d[5]. The actual figure is likely to be more. With the industrial growth and GDP growing at about 8-9% per year, water consumed by industry is likely to be even higher than the previous year and thus the generation of wastewater too. Thus the largest source of marginal water for agriculture is the municipal wastewater in other words the urban wastewater. This water often contains higher levels of salts such as bicarbonates, sodium, chlorides, and sulfates than fresh water that may reduce plant performance and yield quality. Treated effluents are also characterized by higher levels of organic matter, total suspended solids (TSS), nutrients, microelements, and microorganisms than the potable water from which they originated, which may affect the irrigated plants[6].Anthropogenic activities have significantly altered the environment throughout the world including mining, industry and agriculture as well as increase of the urbanization level. For example, on one hand many chemical fertilizers from various ore that cannot be regenerated are decreasing gradually. On the other hand, however, the discharge of wastewater containing abundant nutrients have been considered as the important cause contributing to the eutrophication of water[7]. Many countries throughout the world are approaching or have already reached the limits of their available water supplies. Since agriculture involves the consumptive use of water and fertilizer, therefore, the use of additional water resources of marginal quality or the waste- water can increase the volume of water available for irrigation and may supplement the nutrient requirement of the crop. Sufficient scope therefore, exists for conservative and sustainable use of water in agriculture and improvement in its equitable distribution among users by adopting a variety of measures such as:● Varietal improvement of crops for higher water use efficiency.● Effective soil water management.● Agronomic management and choice of the crops with low water budget.● Need based or demand driven irrigation.● Using water of marginal quality.Thus the present study was aimed to evaluate the effect of urban waste water along with different phosphorus levels on plant morphology, fresh and dry yield, photosynthetic activity and yield of the crop. In addition the heavy metal contents of the waste water and soil samples were also analyzed however, the microbial characteristics were determined only for wastewater.
2. Materials and Methods
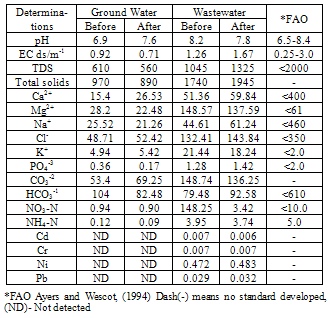
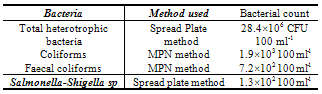
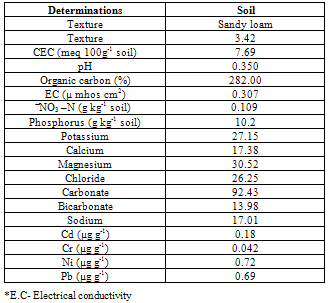
- The urban wastewater includes the wastewater from the households and sewage together with wastewater from local industries of locks and electroplating as well and was collected from the outskirts nearly 5 km from the town where it is already being used to irrigate the crops by the local farmers. Thus pot experiment was carried out in the naturally illuminated net house of the department of Botany Aligarh Muslim University, Aligarh, and each treatment was set simultaneously in triplicate using a completely randomized block design, with four different phosphorus treatments being watered by tap water (GW) and urban wastewater (50%WW and 100%WW). The surface of the seeds was disinfected with 0.01% aqueous solution of mercuric chloride followed by repeated washing with double distilled water (DDW). The disinfected seeds were then inoculated with specific Rhizobium and were sown in earthen pots (10 inch diameter), filled with sandy loam soil mixed with farmyard manure. Tap water (GW) and urban wastewater (WW) characteristics were monitored at the beginning and before the end of experiment. Water samples were collected just before irrigation and were analyzed for physico-chemical characteristics adopting the procedures as outlined in the Standard Methods[8] (Table 1). The soil samples of the experimental pots were collected before the start of the experiment. These samples were also analyzed for standard physico-chemical properties according to Jackson[9] and Ghosh et al.,[10] (Table 3). Microbiological analysis was also carried out and the mean values were obtained from three random samples (Table 2). Heavy metals (Cd, Pb, Ni, Cr) in the water and the soil samples were also analyzed using the atomic absorption spectrophotometer (Table 1 and 3).
|
|
|
3. Data analysis
- The data recorded from the experiment was subjected to two way analysis (ANNOVA) using SPSS software package and the means were compared following the method given by Gomez and Gomez[15].
4. Results and Discussion
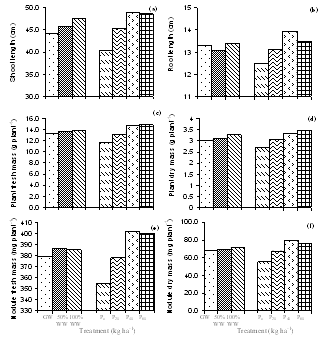
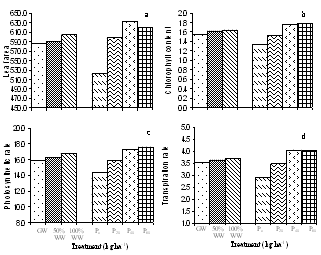
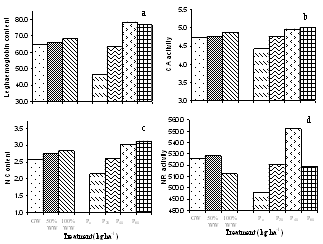
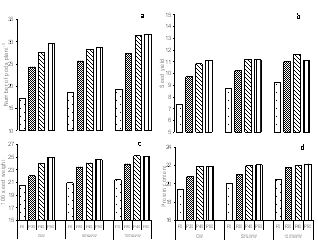
- The analysis of the urban wastewater has revealed that it was alkaline and the average EC, pH, TDS and the observed nutrients including some heavy metals also were within the permissible limits of FAO guidelines for irrigation water quality except for Mg2+ and K+[16] though all were higher than that of ground water. The major effect of EC and TDS on crop productivity is the inability of the plants to compete with ions present in the soil for water while the chloride content of 143.84 mg l-1 was also comparatively low and may not cause toxicity problems. When nutrients are added through effluents and they are either equal or less than the demand of the crop, the build up in the soil is normally minimal. There are reports where it has been shown that the continuous use of effluents may result in the accumulation of nitrate in the soil[17] and is subject to move to down layers which may ultimately result in the contamination of ground water. Similarly NPK fertilizers added at the rate higher than the rate of crop removal accumulate in the soil therefore, a balance between the wastewater and fertilizer dose has to be identified. Although in various studies, dilution has been described as an effective means to minimize toxicity however, under current experimental conditions since the source of irrigation was the urban sewage wastewater which was sufficiently diluted repeatedly due to the mixing of the household wastewater did not cause any toxicity problems. The wastewater contained considerable amount of nutrients which are considered essential for maintaining the soil fertility as well as for enhancing the plant growth and productivity. Wastewater in general proved beneficial in increasing the plant growth characteristics and dry matter accumulation was higher in plants receiving it as a source of irrigation water compared to those receiving ground water (GW). 100%WW recorded much more dry mater among the two concentrations of water used. This is primarily due to the retention of the effluent components mainly due the incorporation of the elements in the dry matter of plants[18] leading to decreasing concentration in the surrounding ground and surface waters. Wastewater contained nitrogen in both nitrate as well as in the form of ammonia and as the vegetative growth includes formation of new leaves, stems and roots, the involvement of N through protein metabolism controls them. This was also clearly indicated by the observed enhanced growth and N content in the leaves under the wastewater irrigation (Fig. 3). Suitability of NH4+ or NO3- for the growth and development of plants depends upon many factors[19]. However, normally the highest growth rate and plant yield are obtained by combined supply of both and therefore, in the present study, the improvement in growth could be due to the cumulative effect of ammonium as well as nitrate ions together. It is noteworthy that applied NH4+-N is toxic for some higher plants including bean and pea[20], however, in presence of NO3--N, it has been reported to benefit sunflower[21], wheat[22] and chickpea[18] and thus the observed nutritional superiority of the wastewater (containing both ammonium-N and nitrate-N) for growth of chickpea was not exceptional and possibly explains the reason of better performance of the crop grown under the its irrigation. Another possible explanation of the increased dry matter is possibly because of the increased leaf area and expansion (Fig. 2) which might have influenced the light absorption within a plant causing stimulation of PN (Fig. 2), thereby optimizing the CO2 assimilation and photosynthetic production. The increase in the leaf area brought about by N supply causing the expansion of the individual leaves was also reported by Taylor et al.[23] and Gastal and Lemaire[24] which may be through its effect on cell division and cell expansion[25] Similarly increased N supply has also been found to enhance the activities of CA and RuBP carboxylase as reported by Terashima and Evans[26] and the stimulation in the activity of CA as observed in the present study (Fig. 3).
5. Conclusions
- Based on these findings we can conclude that the urban wastewater proved beneficial for the crop growth and productivity. The wastewater proved an effective source of essential nutrients and reduced the quantity of phosphorus doses and thus acted not only as a source of irrigation water but of nutrients also. Although the presence of some microbes could be a cause of concern but since in our case the crop is eaten only after cooking so it does not seem to pose any harm. Also the presence of heavy metals were detected in wastewater but it does not seem to have affected the crop much but its continuous usage may result in their buildup to phytotoxic level and thus regular monitoring is required for the safe irrigation and benefits, so that its reuse can effectively contribute to fill the increasing gap between water demand and water availability.
ACKNOWLEDGEMENTS
- Hamid Iqbal Tak thanks the NWU for awarding the Post-doctoral research fellowship.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML