-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2012; 2(1): 51-58
doi:10.5923/j.plant.20120201.08
Phytochemical Constituents and Antimicrobial and Grain Protectant Activities of Clove Basil (Ocimum gratissimum L.) Grown in Nigeria
Abdullahi Mann
Department of Chemistry, Federal University Technology, Minna, P. M. B. 65, Niger State, Nigeria
Correspondence to: Abdullahi Mann, Department of Chemistry, Federal University Technology, Minna, P. M. B. 65, Niger State, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Ocimum gratissimum Linn (Lamiaceae) is an herbaceous plant reputed for many medicinal and agronomic practices amongst Nigerian peasant farmers. O. gratissimum was investigated for antimicrobial activity against ten micro-organisms and for grain protectant activity against Callosobrochus maculatus. The phytocontituents of the aerial part of O. gratissimum were extracted with 95% ethanol using the percolation method. The crude ethanol extracted was further fractionated into hexane, chloroform and methanol fractions. The fractions obtained were screened for phytoconstiteunts, antimicrobial and grain protectant properties. Result showed that hexane fraction exhibited the highest antimicrobial activity against Vibrio cholera and Klebsiella pneumonia. Similarly hexane fraction also showed the highest grain protectant activity. The other extracts of the O. gratissimum did not significantly inhibit both bacterial growth and grain infestation. However, the methanol fraction contains phytocompounds such as phenolic compounds associated with antioxidant properties. The study shows that O. gratissimum extractants are potential sources of antimicrobial and preservative agents.
Keywords: Antimicrobial Activity, Grain Protectant Activity, Ocimum Gratissimum, Weevil Perforation Index, Callosobruchus Maculatus, Traditional Medicine, Phytoconstituents
Cite this paper: Abdullahi Mann, Phytochemical Constituents and Antimicrobial and Grain Protectant Activities of Clove Basil (Ocimum gratissimum L.) Grown in Nigeria, International Journal of Plant Research, Vol. 2 No. 1, 2012, pp. 51-58. doi: 10.5923/j.plant.20120201.08.
Article Outline
1. Introduction
- Traditional medicine continues to provide health coverage for over 80% of the world population, especially in the developing world[1]. Plants are the major constituents of traditional medicine[2]. Many of the plant materials used in herbal medicine are readily available in rural areas and this has made it relatively cheaper than orthodox medicine[3]. The upsurge in the prevalence of side effects of many synthetic antimicrobial agents and incidence of multi-drug resistant bacteria and pests has spurred scientists onto the research for plant based antimicrobial of therapeutic and pesticidal potentials[4-7]. Ocimum gratissimum Linn (Lamiaceae) is an herbaceous shrub notably found in tropical countries including Nigeria, where it is commonly called Clove basil, Sweet basil, teabush, Scent leaf or fever plant; but it is also popularly known with different local names in Nigeria (Nupe: Tanmotsungi-wawagi; Ebira: Ireru; Hausa: Dai doya ta gida; Yoruba: Efinrin ajase; Ibo: Nchanwu)[8-10].Many species of the genus Ocimum namely: Ocimum americanum, Ocimum basilicum, Ocimum canum, Ocimumgratissimum, Ocimum sanctum and Ocimum suave have been reputed for various medicinal uses[10-13].Several ethnobotanical surveys show that Ocimum gratissimum was among the plants reported in Nigeria communities to be used traditionally to treat bacterial infections such as enteric diseases viz: diarrhoea, dysentery and other gastrointestinal infections; upper respiratory tract infections associated with coughing pneumonia, asthma and bronchitis; urogenital infections including sexually transmitted diseases, skin infections (dermatitis, eczema, scabies), wounds and ulcers; headache, ophthalmic, insect bites, nasal bleeding, stroke, measles, paludism; and bacterial fevers such as typhoid fever and diabetes and veterinary problems[13-36]. It is also used in the treatment of epilepsy, shigellosis, trypanosomiasis, convulsion, pile and anaemia in Nigeria[37]. It is also implicated in the oral hygiene and veterinary in Nigeria[38, 39].Comprehensive biological activities of O. gratissimum has been reviewed[14] and it is associated with antibacterial, antifungal, hypoglycaemic, antipyretic, anti-nociceptive, antioxidant, anti-inflammatory, anthelmintic, chemo- preventive, anti-carcinogenic, free radical scavenging, radio protective, antidermatophytic activities, and numerous others pharmacological use[40-61]. Earlier reports have also shown the smooth muscle contracting and antimutagenic activity[62] as well as its anti-diarrhoeal effects in experimental animals[63], high antiviral indices against HIV-1 and HIV-2[64]; shigellocidal properties[65,66], anti- trypanosomal effects[67], immunobiologicalactivity[68], gastro- protective properties[69], controlling agent for food spoilage and mycotoxin producing fungi[70], disintegrant properties of its seed mucilage[71], and as a relaxant on isolated ileum from guinea pig[72]. Its essential oil has mosquito repellant, insecticidal properties[73,74]. The essential oil of O. gratissimum and its main component eugenol were reported to be efficient in inhibiting Haemonchus contortus[75,76]. Currently, basil is mainly used as a culinary herb as well as perfumes and cosmetics[77]. It is therefore important that phytochemical composition be correlated to the antimicrobial activity in order to verify the therapeutic value proclaimed by the traditional healers.One major factor responsible for promoting grain production in developed countries has been attributed to the usage of insecticides in grain protection and storage. Many plant components are now known to possess herbicidal, insecticidal or fungicidal properties[78-81]. Preparations made from Nigerian plants are identified with pesticidal activities[82-88]. The discovery and development of these products were based on the significance of the Nigerian plants in folklore medicine and agronomic practices amongst our peasant farmers. Peasant farmers in northern Nigeria indigenously use various plants to protect cereals and legumes against pest damage during storage with O. gratissimum being one of such plants[7]. It is based on this view that, bioassay screening method[87] was adopted for screening Ocimum gratissimum for grain protectant activities. Despite these scientific and medicinal values, comparative analyses of its phytochemical evaluation vis-à-vis the antimicrobial and pesticidal potentials have not been investigated. Therefore, the present study reports phytochemical, antimicrobial and grain protectant activities of O. gratissimum used for the treatment of human infections and agronomic pests.
2. Materials and Methods
2.1. Collection and Identification of Materials Used
- The aerial parts of O. gratissimum were collected from Kuchi-gbako, a village along Bida-Doko road of Lavun Local Government, Niger State as described by the traditional healers and farmers. The plant was botanically identified by Mal Muazzim Ibrahim of the Herbarium Unit of the Department of Medicinal Plant Research and Traditional Medicine, National Institute for Pharmaceutical Research and Development, Garki – Abuja, Nigeria where voucher specimen (No. NIPRDH 1285) was deposited. Callosobruchus maculatus was obtain from national cereal research institute, Baddegi, Niger State and maintained on seeds of the cowpea[Vigna unguiculata (L.) Walp] cultivar life brown[87].
2.2. Extraction Procedure
- The plant material was air-dried under the laboratory room conditions for one week and then milled into coarse powder by using clean mortar and pestle. The powdered material (200g) was percolated with 95% ethanol (2.5L) for two weeks. The extract was filtered and evaporated to dryness using a rotavapor to give a dark greenish residue (43.5g)[89].
2.3. Test procedure for Antimicrobial Activity
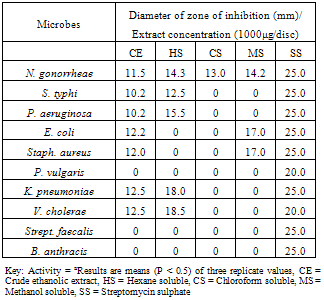
- The crude ethanol extract of the aerial parts of O. gratissimum was screened in vitro for antimicrobial activity against ten pathogenic microorganisms (Table 2) using Agar-dilution streak technique[90] as follows: the test organisms were prepared by incubating them in freshly prepared nutrient broth at 37oC for 8 h the cultures were serially diluted with sterile normal saline. 48 mg of the test extract was dissolved in 1ml of absolute ethanol and made up to 3ml with sterile distilled water to give a concentration of 16 mg/ml of extract. 1ml of the prepared extract was then introduced into 15 ml of molten Agar placed in water at 54 oC these were mixed well and poured into sterile petri-dish plates to give a concentration of 1000 µg/ml of Agar. Other concentrations were similarly prepared. The plates were then hardened in a refrigerator for 15 min. Thereafter thestandardized test organisms (1000 ml each) were inoculated onto the nutrient Agar plate and incubated at 37℃ for 24-48 h the results of the tests done in triplicate are shown in Table 2.
2.4. Fractionation of Extracts
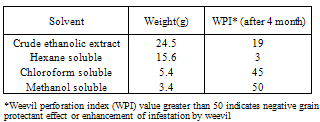
- The crude extract (100 g) was extracted with hexane and 70% aqueous methanol (150 ml, 1:1), hexane soluble fraction was separated and concentrated in vacuo to give a hexane residue (15.56 g), and the methanol layer was then extracted with chloroform (150 ml). The resultant chloroform soluble and methanol soluble portions were separated and concentrated in vacuo to give chloroform residue (5.4 g) and methanol residue (3.2 g) respectively.
2.5. Phytochemical Screening of Extracts
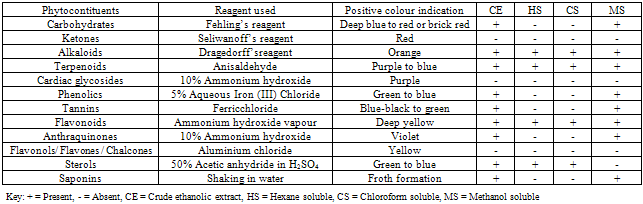
- The plant extracts were phytochemically screened using standard techniques for the detection of Sterols, saponins, phenolics, tannins, flavonoids, terpenoids andalkaloids[91- 93].
2.6. Cowpea Weevil Bioassay with the Plant Extracts
- The residues obtained from the fractionation of O. gratissimum were screened for grain protectant activity against C. maculatus using cowpea weevil bioassay techniques[87] as follows: Unperforated cowpea seeds (50 g) from newly harvested dry pods were weighed out and from 10 g were transferred to each of four Erlenmeyer flasks. The cowpea seeds in each of these three flasks were separately treated with the various extracted residues (1 g) each. The untreated seeds in the fourth flask served as a control.Freshly emerged adults of C. maculatus (age, 0-8 h) were used to infest the cowpea seeds in each flask. The flasks were covered with mesh net and left on the shelf at room temperature. The control and the three treated samples are triplicated. After 4 months the cowpea seeds in each flask were examined for perforations. The number of cowpea seeds perforated in treated and control were counted for determination of weevil perforation index (WPI). The weevil perforation index, defined as percentage of treated cowpea seeds perforated X 100/percentage of control cowpea seeds perforated + percentage of treated cowpea seeds perforated, was calculated for each extract for comparison of grain protectant properties of O. gratissimum.
2.7. Test Organisms
- Stock culture of Neisseria gonorrheae, Salmonella typhi, Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, Proteus vulgaris, Klebsiella pneumoniae, Vibrio cholerae, Streptococcus faecalis and Bacillus anthracis were obtained from Microbiology Laboratory, Federal University of Technology, Minna. These cultures were checked for viability and purity and maintained on nutrient agar slopes.
2.8. Statistical Analysis
- The data was analyzed with ANOVA and the means were separated using Duncan Multiple Range Test at a probability level of 5%.
3. Results and Discussion
- The crude 95% ethanol extract of O. gratissimum has demonstrated antimicrobial activity against N. gonorrhoeae S. typhi, P. aeruginosa, K. pneumoniae, and V. cholera at a concentration of 1000 µg/ml (Table 1). Most of the fractions obtained had exhibited broad-spectrum antimicrobial activity for many test organisms at 1000 µg/ml which is a good inhibitory concentration. Since crude extracts with activity at concentrations of 1000 µg/ml and below are considered as promising bioactive agents for further work[94].
|
|
|
4. Conclusions
- The preliminary biological and phytochemical screenings of O. gratissimum results are quite promising and have strongly indicated the grain protectant property as well as the antimicrobial activity spectra of aerial parts of the plant. The present result also showed the possible phytocompounds to which the biological activity may be attributable. Further work is ongoing to isolate and elucidate the structure of the bioactive compounds and to screen its pure active constituents against agronomic pests.
ACKNOWLEDGEMENTS
- The author wishes thank the traditional healers and farmers consulted for supplying the ethnobotanical information of the plant. We are also grateful to Mal Muazzim Ibrahim of the Herbarium Unit of the Department of Medicinal Plant Research and Traditional Medicine, National Institute for Pharmaceutical Research and Development, Garki – Abuja, Nigeria for identifying the plant as well as the authorities of the National Cereal Research Institute, Baddegi, Niger State, Nigeria for providing authenticated cowpea seeds, Vigna unguiculata (L.) Walp; and weevils, Callosobruchus maculatus (L.) used in the grain protectant screening.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML