-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2012; 2(1): 9-14
doi:10.5923/j.plant.20120201.02
The Inhibitive Effect of Extract of Flowers of Cassia Auriculata in 2 M HCl on the Corrosion of Aluminium and Mild Steel
A. Rajendran1, C. Karthikeyan2
1Department of Chemistry, Sir Theagaraya College,Chennai, Tamil Nadu, 600021, India
2Research and Development Centre, Bharathiar University, Coimbatore, 641046, Tamil Nadu
Correspondence to: A. Rajendran, Department of Chemistry, Sir Theagaraya College,Chennai, Tamil Nadu, 600021, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Polyphenolics of air dried flowers of Cassia auriculata was tried as a corrosion inhibitor on Aluminum and Mild steel in 2M HCl at 30 ± 1℃ by weight loss, polarization study and impedance methods. It was ascertained that the percentage of inhibition increases with the increase in concentration of the extracts but decreases with an increase in temperature. The inhibitive effect of the extract of flowers of Cassia auriculata could be attributed to the presence of some phytochemical constituents in the extract which is adsorbed on the surface of the Aluminum metal and Mild steel.
Keywords: Acid Solutions, Aluminum, Mild Steel, Electrochemical Calculation, Weight Loss, Acid Corrosion
Cite this paper: A. Rajendran, C. Karthikeyan, The Inhibitive Effect of Extract of Flowers of Cassia Auriculata in 2 M HCl on the Corrosion of Aluminium and Mild Steel, International Journal of Plant Research, Vol. 2 No. 1, 2012, pp. 9-14. doi: 10.5923/j.plant.20120201.02.
Article Outline
1. Introduction
- Corrosion is a natural phenomenon, which can be considered either chemical or electrochemical in nature, degrades the metallic properties of metal and alloys make them unfit for specific role. Corrosion of metals is a major industrial problem that has attracted much investigations and researches. This is because some industrial processes such as acid cleaning, pickling and etching facilitate contact between metal and aggressive medium (such as acid, base or salt), consequently the metal is prone to corrosion. In order to reduce the menace due to corrosion of industrial installations, several steps have been adopted. However, one of the best options available for protecting metals against corrosion involves the use of corrosion inhibitors. Corrosion inhibitors are widely used in industry to reduce the corrosion rate of metals and alloys in contact with aggressive environment. Most of the corrosion inhibitors are synthetic chemicals, expensive and very hazardous to environment. Therefore, it is desirable to source for environmentally safe corrosion inhibitors[1– 7]. Most of the best known corrosion inhibitors are organic compounds which contain electronegative functional groups and pi-electrons in triple or conjugated double bonds. For this class of inhibitors, the presence of heteroatoms (such as S, N, O and P) as well as aromatic rings in their structures is the major adsorption centre. Several inhibitors have been synthesized and used for the inhibition of the corrosion of mild steel in acidic medium but some of these inhibitors are not environmentally friendly .It has been shown that natural products of plant origin contain different organic compounds (e.g. alkaloids, tannins, pigments, organic and amino acids, and most are known to have inhibitive action . Literature survey has shown that the inhibitive effect of some plant’s solution is due to the adsorption of molecules of phytochemicals present in the plant on the surface of the metal[8-11], which blocks the metal surface and thus do not permit the corrosion process to take place. The inhibition of corrosion by Cassia auriculata was attributed to the presence of arabinogalactan, oligosaccharides, polysaccharides and glucoproteins since these compounds contain oxygen and nitrogen atoms which are the centers of adsorption. The encouraging results obtained by this research permit to test more plant materials.Green corrosion inhibitors are cheap, biodegradable and do not contain heavy metals or other toxic substances. The successful uses of naturally occurring substances to inhibit the corrosion of metals in acidic and alkaline environment have been reported by some research groups. On this note, ethanol or aqueous extracts of some plants have been found to be good corrosion inhibitors for some metals (notably Aluminium, Mild steel and zinc). Therefore, the present study is aimed at investigating the corrosion inhibit properties of ethanol extracts of flowers of Cassia auriculata for Aluminium and Mild steel in 2 M HCl. Aluminium is a soft, durable, lightweight, malleable metal with appearance ranging from silvery to dull grey, depending on the surface roughness. Aluminium is nonmagnetic and non sparking. Aluminium and its alloy are recommended for building purpose and for various internal outfits. Aluminium is remarkable for its low density and for its ability to resist corrosion to some extent due to the phenomenon of passivation. But its corrosion takes place in aqueous acidic conditions[12].Mild steel finds application in many industries due to its easy availability, ease of fabrication, low cost and good tensile strength besides various other desirable properties. It suffers from severe corrosion when it comes in contact with acid solutions during acid cleaning, transportation of acid, de-scaling, storage of acids and other chemical processes. Some chemicals as corrosion inhibitor are currently used in industry to prevent or to reduce the corrosion rates of metals in acid media. Due to toxic nature and high cost of these chemicals[13-17] it is necessary to develop environmentally acceptable and less expensive inhibitors. Flavonoids are the naturally occurring chemicals present in plant kingdom. These commonly occur as Flavonoid-O- glycoside in which one of the Flavonoid hydroxyl groups is bound to sugar or sugars by an acid labile hemiacetal bond. Recently, it has been reported that the Flavonoids extracted from the certain plants are found to have profound corrosion inhibition activity to certain metals like iron, Aluminium and some alloys like steel, etc., the present work aims at the study of the Flavonoids obtained from the flowers of Cassia auriculata, which are easily available in the southern region of India[18]. The recent trend in corrosion chemistry is towards environment-friendly inhibitors. Most of the natural products are non toxic, biodegradable and readily available in plenty. Various parts-seeds, fruit, leaves, flowers etc., have been published on the use of natural products as corrosion inhibitors. Tannins are being used for the protection of steel against corrosion in cooling water systems and in paints (wash primers)[19]. Since they are ecologically harmless, natural tannins are often used in corrosion-preventing primers for surface treatments of steel. Tannins are poly phenols capable of complexing iron by forming a chelate[20,21]. So, we consider medicinally valuable plant Cassia auriculata which belongs to Fabaceae family. It is a large spreading shrub with irregular angled, glabrous branches. The flowers are bright yellow in color. This plant contains many useful chemical constituents. The parts of (leaves, flowers and so on) Cassia auriculata have many medicinal properties like antibacterial, antioxidant, antidiabetic, antiulcer, anthelmintic, anticancer, liver injury etc.[22-25].
2. Experimental Section
2.1. Extraction Method
- Fresh flowers of Cassia auriculata were collected from the area called Manapparai, Trichy during January, 2010 and they were extracted with 80% alcohol under reflux. The concentrates were successively fractionated with Petroleum ether 60-80 (3×150 ml), Peroxide free Et2O (3×250 ml) and Ethyl acetate (4× 250 ml). The petrol fraction and other fraction did not yield any isolable solid. Using the alcoholic extract, the following characterization was done.
2.2. Characterization
2.2.1. Color of the Extract
- The color of the extract after filtration was noted visually. Density was measured by specific gravity method. PH was determined by the glass electrode.
2.2.2. Preparation of Buffer solution
- The pH 4.0 buffer powder (Capsule, Nice laboratory, Cochin,) was dissolved in 100 ml distilled water to give a solution having pH 4.0 at 20℃.
2.3. Identification of the Flavonoids
2.3.1. Fluorescence Test and Ammonia Vapor test
- A Ultra-violet lamp equipped with two 1.5 watt Black-ray tubes and covered with a glass plate is used for viewing the developed chromatograms. The developed chromatogram was shown to the mouth of the ammonia solution bottle and then viewed under UV light.
2.3.2. Paper Chromatogram
- A strip of Whatmann No.1 filter paper, about 25-30 cm long and 1.5 cm wide was marked lightly with a pencil line about 5 cm from one end. The plant extract was spotted from a capillary pipette on to a spot marked in the middle of the pencil line. The solvent was allowed to evaporate. The paper was allowed to hang in the gas jar with the upper end held in the glass trough. The solvent BAW (n-Butanol, Acetic acid, Water in the ratio 4:1:5) was introduced to saturate the air in the gas jar. The paper was introduced in to the glass trough and the gas jar closed. The solvent moves by capillary action in to the paper and development proceeds. After the front of the solvent had moved to upper edge of the paper, the gas jar was opened, the paper removed, and position of the front marked. The Rf value was found.
2.3.3. Ultraviolet Spectroscopy
- The spot was cut, dissolved in methanol and after the evaporation of the solvent and the residue obtained was dissolved in spectral grade methanol and UV spectrum of the product recorded in methanol and sodium methoxide.
2.4. Materials
- Aluminium alloy specimens having weight percentage composition as follows; Si-0.362%, Fe-0.549%, Cu-0.077%, Mn-1.219%, Ti- 0.026%, Pb-0.063%, Zn-0.004% and the remainder being Al were used. The specimens were of dimensions 2 cm x 2 cm and thickness 1.32 mm. The alloy specimen were polished mechanically using SiC emery papers of grade numbers 220, 400 and 600, then washed thoroughly with distilled water and degreased with ethanol and acetone, air dried before being immersed in the acid solution. The blank corrodent was 2 M HCl solution. Stock solutions of the plant extract were prepared by boiling weighed amounts of the dried and ground plant material for 4 h in ethanol). The solutions were cooled and then filtered and stored. From the stock solutions, inhibitor test solutions were prepared in the concentration range of 0.1 - 0.5 g/L using excess acid as solvent at room temperature and 60℃ using water bath.
2.5. Weight Loss Method
- The aluminium and mild steel coupons were weighed and placed vertically in 60 ml of aerated, unstirred 2M HCl with and without the inhibitor for four hours. The coupons were removed from the solution and they were cleaned by brushing under running tap water to remove the corrosion products, dried. The cleaned and dried specimens were weighed before immersion in the respective test solutions of 2 M HCl using JA 1003A electronic weighing balance with the accuracy of ±0.005. At the end of the tests, the specimens were carefully washed in absolute ethanol and then reweighed. Triplicate experiments were performed in each case and the mean values reported. The percentage inhibition efficiency (% I) was calculated using the following equation % I = Wo - Wi / Wo × 100Where Wo and Wi are the weight losses in uninhibited and inhibited corroding solutions respectively.
3. Results and Discussion
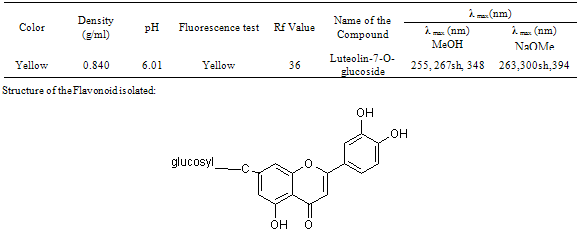
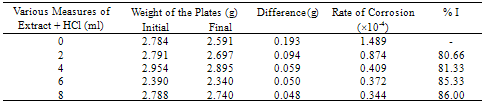
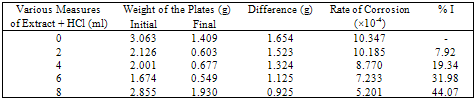
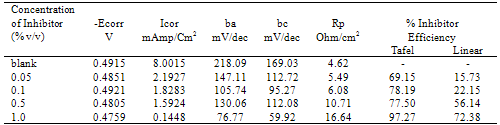
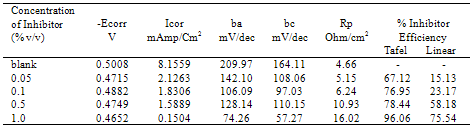
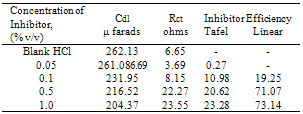
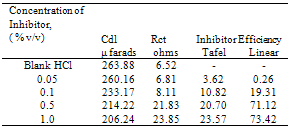
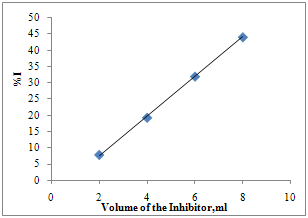
- The important characteristics of the extracts obtained from the plant Cassia auriculata such as the color, density, pH, color under fluorescence test, Rf value obtained from paper chromatographic technique, the name of the Flavonoid compound and its UV-Visible spectral characteristics (λ max (nm) in MeOH and NaOMe) are presented in Table 1. From the chromatographic and spectral characteristics, the Flavonoid compounds obtained from Cassia auriculata was found to be Luteolin-7-O-glucoside.Table 2 displays the results obtained from the corrosion studies carried out using the extract (ethyl acetate fraction) of Cassia auriculata on the mild steel in 2M HCl at 30 ± 1℃. The kinetics and mechanism of corrosion of mild steel in 2M HCl at 30 ± 1℃ containing the extract of Cassia auriculata was studied by weight loss and polarization studies. From the results it is understood that the corrosion rate is decreased considerably or the % inhibition (I %) of corrosion increases in the presence of traces of compounds obtained from the plant on both mild steel and Aluminium metal Figure 1 The % inhibition efficiency (I %) of various concentration of a myricetin - Fe2+ and rutin - Fe2+system in controlling corrosion of mild steel in an aqueous solution containing the extract of Cassia auriculata was evaluated by weight loss study. It is reported that a synergistic effect was seen between myricetin / rutin and Fe2+. A similar synergistic effect is believed to exist between Luteolin and Fe2+[26]. The transport of inhibitors towards the metal surface plays a major role in controlling corrosion of mild steel. Formation of micelles by surfactants changes the % inhibition. The % inhibition decreased as the period of immersion increased. This may be due to the fact that a kind of protective is formed over the surface of base metal. The protective film was analyzed by UV spectroscopy. The film consisted complex and iron hydroxide. The film was found to be UV fluorescent. Corrosion inhibition studies on aluminium in acidic medium (2M HCl) at 30 ± 1oC were also investigated by weight loss and polarization studies. The results are presented in Table 3. The corrosion rate is decreased considerably or the % inhibition (I %) of corrosion increases in the presence of traces of compounds obtained from the plant Figure 2. This is attributed to the fact that the active principal constituents of the natural products form protective film on the metal surface by coordinating with the metal ion through O, S and N atoms of the functional groups present in the active principle constituents. When the active principal constituents are extracted with acids or organic solvents or with water, usually a mixture of inhibitors present in the plant extract may show synergistic effect. From the close examination of %I of Tables 2 and 3, it is concluded that the extract obtained from the flowers Cassia auriculata almost inhibit the corrosion of mild steel and Aluminium to an extent(80%) and 40% respectively. Hence, it is concluded that the extract obtained from Cassia auriculata can be used as a corrosion inhibitor for mild steel and Aluminium in 2M HCl.
 | Figure 1. % corrosion inhibition of the extract of Cassia auriculata on Mild steel in 2M HCl at 30 ± 1℃ |
 | Figure 2. % corrosion inhibition of the extract of Cassia Auriculata on Aluminium metal in 2M HCl at 30 ± 1℃ |
|
|
|
|
|
|
|
 | Figure 3. Temperature–time curves for Aluminium and Mild steel corrosion in 2 M HCl in the presence of extract of Cassia auriculata in different concentrations |
4. Conclusions
- The Rf value obtained from paper chromatographic studies in BAW (4:1:5) is 36. Thus the Flavonoid present was found to be Luteolin which also supported by the λmax value of UV.Phytochemical constituents (like Luteolin) in the extract, adsorbed on the surface of the Aluminum and Mild steel.A synergistic effect is believed to exist between Luteolin and Fe2+ like in the case of myricetin/rutin and Fe2+.The transport of inhibitors towards the metal surface plays a major role in controlling corrosion. Then, further studies going…
ACKNOWLEDGEMENTS
- The author AR gratefully acknowledges the funding support rendered by the University Grants Commission, for his major research project [F.No 35-147/2009(SR)]. He thanks the principal and the management of , Chennai-21 for the constant encouragement given.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML