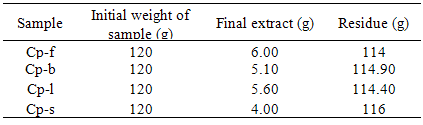-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Plant Research
p-ISSN: 2163-2596 e-ISSN: 2163-260X
2011; 1(1): 29-33
doi: 10.5923/j.plant.20110101.05
Phytochemical Screening and Biological Activity of Calotropis Procera (Ait). R. Br. (Asclepiadaceae) Against Selected Bacteria and Anopheles stephansi Larvae
Hiren Doshi 1, Hitesh Satodiya 1, Mukund Chandra Thakur 2, Farzin Parabia 2, Arif Khan 2
1Dept. of Pharmaceutical Chemistry
2Dept. of Biotechnology; Ashok & Rita Patel Institute of Integrated Study & Research in Biotechnology and Allied Sciences, New Vallabh Vidyanagar, 388121, Gujarat, India
Correspondence to: Hiren Doshi , Dept. of Pharmaceutical Chemistry.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The ethanol extracts of flowers, young bud, mature leaves and stems of Calotropis procera (Ait). R. Br. (Asclepiadaceae) was screened for phytochemical properties, antimicrobial (agar dilution method) activity and effectiveness on third instar larvae of Anopheles stephansi. Qualitative estimation of alkaloids, carbohydrates, glycosides, saponins, proteins, fixed oils, starch, triterpenoid, phenolics and tannins showed their presence in almost all the plant part extracts. While, gum and mucilage were absent in all the plant extracts. Quantitative estimation of different parts of the plant extracts had large quantity of carbohydrate and tannin in flower while young buds had higher amount of phenolic compounds and oil. Mature leaves showed maximum activity against all the bacterial strain used in the study. The extracts of mature leaves showed highest activity of 100% mortality at 2000 ppm after 48 hours of incubation against 3rd instar larvae of A. stephansi. LD50 and LD90 values suggested that mature leaves of C. procera had higher mortality rate against larvae of A. stephansi.
Keywords: Anopheles Stephansi, Calotropis Procera, Larvicidal Activty, LD50, LD90, Microbial Activity & Plant Extracts
Cite this paper: Hiren Doshi , Hitesh Satodiya , Mukund Chandra Thakur , Farzin Parabia , Arif Khan , "Phytochemical Screening and Biological Activity of Calotropis Procera (Ait). R. Br. (Asclepiadaceae) Against Selected Bacteria and Anopheles stephansi Larvae", International Journal of Plant Research, Vol. 1 No. 1, 2011, pp. 29-33. doi: 10.5923/j.plant.20110101.05.
Article Outline
1. Introduction
- Plant produces a wide range of bioactive molecules via secondary metabolic pathways. Most of these molecules have been developed on the basis of traditional knowledge in health care and in many cases, there is a correlation between the indications of pure substances and those of respective crude extracts used in traditional medicine[1]. Plants are important source for the discovery of novel pharmacologi-cally active compounds. Many drugs are derived directly or indirectly from plants[2] which are used as antimicrobial and antifungal agents[3]. Despite the advances in antimicrobial therapies, many problems remained to be solved for the most antimicrobial drugs available[4].In many developing countries, malaria and other vector-borne diseases are of major concern due to improper sanitation, inappropriate treatment and devoid of access to clean water[5]. Malarial contributes to the major disease in India[6]. One of the methods to control is to control thevectors for eradication of disease transmission. Use of synthetic insecticides to control the insect pests has resulted in development of resistance in some vectors of malaria, filariasis and dengue fever[7]. In last few decades, the findings of various natural plant products against mosquito vectors have proved to be an alternative to the synthetic chemicals[8-15]. Anopheles stephansi Linn. (Diptera, Culicidae), the vector which transmit malaria are disseminated everywhere within the world. In addition, A. stephansi population is highly resistant to insecticides[16]. It would be of great relevance to search for alternatives in combating malaria and proliferation of A. stephansi. Many natural compounds have been suggested as alternatives against conventional chemical control[17]. The genus Calotropis has attained a high repute for its various medicinal properties[18, 19]. The plant C. procera belonging to the family Asclepiadaceae was selected for the present work. This large family comprises of around 175–180 genera and 2200 species distributed in the tropical and subtropical region. Many of which possess biologically active compounds[20]. Calotropis is a small genus having 6 species of shrubs or small trees, distributed in tropical and subtropical Africa, Asia and America. Two species namely C. procera and C. gigantae are found in India which closely resembled to each other in structure and in functional uses[21].
2. Materials and Methods
2.1. Collection and Identification of Plant Material
- Fresh Flowers, young buds, mature leaves and stem of C. procera were collected from Vallabh Vidyanagar and neighbouring farm areas of Anand district, Gujarat. The samples were clean and packed in polythene bag separately. Flowers, young bud, mature leaves and stems of C. procera are abbreviated as Cp-f, Cp-b, Cp-l and Cp-s respectively.
2.2. Extraction of Phytochemicals
- Plant materials (Cp-f, Cp-b, Cp-l and Cp-s) were washed with running tap water followed by distilled water. The samples were blotted and dried with the help of absorbent towels and cut into small pieces. The fresh plant materials were processed for ethanol extraction using soxhlet apparatus. The extracts were filtered through Whatman no.1 filter paper, concentrated under vacuum and stored at 10 – 15°C for further use.
2.3. Phytochemical Screening
2.3.1 Qualitative Analysis
- All ethanol extracts plant materials (Cp-f, Cp-b, Cp-l and Cp-s) were analysed for alkaloids, carbohydrate, glycosides, saponins, proteins, phytosterols, phenolic compounds, tannins, gum, mucilage as described by Raman[22].
2.3.2. Quantitative Estimation
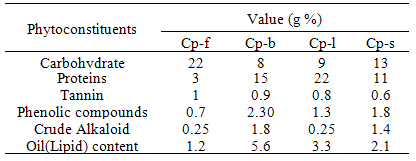
- The ethanol extracts of plant materials (Cp-f, Cp-b, Cp-l and Cp-s) were subjected to the quantitative phytochemical screening for total carbohydrate[23], total protein[24], tannins & phenolic compounds[25], crude alkaloid[26] and oil (lipid)[27].
2.4. Determination of Antibacterial Activity
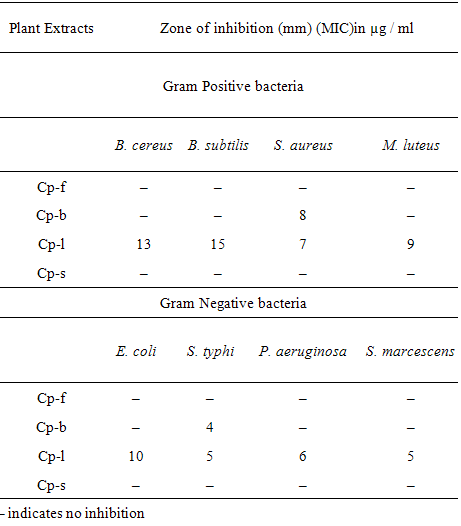
- In present study, pure culture each of four Gram positive (Bacillus cereus, B. subtilis, Staphylococcus aureus, Micrococcus luteus) and Gram negative bacteria (Escherichia coli, Salmonella typhi, Pseudomonas aeruginosa, Serratia marcescens) were obtained from MTCC Chandigarh. Antibacterial susceptibility testing was carried out by agar disc diffusion method[28]. The uniform growth rate was maintained on nutrient broth (Hi Media, pH 7.4) at 1x108 cfu/ml. The bacterial culture was compared with 0.5 Mc Farland turbidity standards, which is equivalent to 1x108 cfu/ml bacterial cell density[28].
2.4.1. Antibacterial Susceptibility Test by Agar Disc Diffusion Method
- The above inoculums of each bacterial strain (200 l) were added to autoclaved nutrient agar plate (Hi media) at a temperature of near about 45°C. Sterile disc (7 mm) was saturated with 5 g of the ethanol extracts at room temperature. The disc was introduced on the upper layer of the seeded agar plate and incubated at 37°C for 24 hours. The zone of inhibition on medium plates was measured as antimicrobial activity. The experiments were performed under strict aseptic conditions in triplicate.
2.5. Efficacy against Larva of A. stephansi
- The efficacy of active principle of different parts of C. procera was evaluated for larvicidal activity against third instar larvae of A. stephansi (vector mosquito). Fresh formulation of extracts ranging from 100 to 5000 ppm was prepared. The larvae of A. stephansi were chosen from the culture and released in 100 ml of test formulation taken in glass beakers in triplicate. A minimum of 25 larvae were exposed at each time for individual plant extract. The beakers were covered with fine muslin cloth and kept at room temperature for 5, 24 and 48 hours. The percentage mortality of larvae was subjected for regression analysis. D50 and LD90 values were also calculated as per the procedure described by Acharya et al.[29].
3. Results and Discussion
- Ethanol extractable values of C. procera are given in Table 1. The higher amount of the extract was obtained in Cp-f sample followed by Cp-l, Cp-b and Cp-s. Therefore, the flower could be the best option for the study of alkaloid content. Phytosterol were observed only in Cp-b and Cp-l samples while flavonoids were present in all the extracts except Cp-s. Gum and Mucilage were absent in all the extracts. Cp-f contained higher amount of carbohydrate and tannin than Cp-b, Cp-l and Cp-s while Cp-b contained larger quantity of phenolic compounds and oil (Table 2). Proteins were more in Cp-l as compared to rest of the plant parts. Our findings are in agreement with the results reported by several researchers[30- 32].
|
|
|
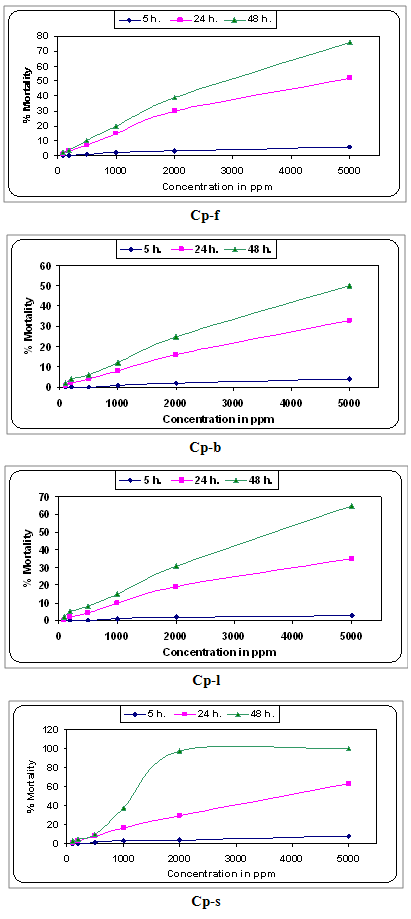 | Figure 1. Effect of plant extract on 3rd instar larvae of Anopheles stephansi at different concentration with respect to time. |
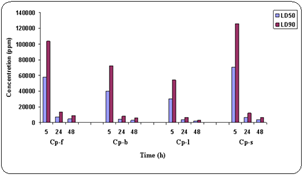 | Figure 2. Comparison of LD50 and LD90 at different concentration with respect to time. |
4. Conclusions
- The plant C. Procera is typically rich in most of the phytochemicals studied. C. procera possesses the antibacterial phytochemicals or toxins as evident by the formation of inhibition zone on the plate surface containing bacterial strain. Therefore, either plants or plant parts could be processed and used against many micro-organisms. The mature leaves of C. procera could be the best option to extract the phytochemicals and their uses against the larvae of A. stephansi.
ACKNOWLEDGMENTS
- We thank Charutar Vidya Mandal, New Vallabh Vidyanagar for providing facility and financial support to undertake the research work. Authors acknowledge Dr. H. C. Srivastava and Mr. Sukhmaya Banerjee, National Institute of Malaria Research (ICMR), Civil Hospital Nadiad, Gujarat, for their technical support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML