-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Public Health Research
p-ISSN: 2167-7263 e-ISSN: 2167-7247
2025; 15(2): 57-64
doi:10.5923/j.phr.20251502.03
Received: Aug. 5, 2025; Accepted: Sep. 12, 2025; Published: Sep. 20, 2025

Diagnostic Performance of Rapid Diagnostic Test, Microscopy, and Polymerase Chain Reaction for Malaria Diagnosis in High-burden States in Nigeria
Abiodun F. Ipadeola 1, 2, Elvis E. Isere 2, 3, Lazarus O. Omenyi 2, Timothy A. Attah 2, Oladipupo B. Ipadeola 4, Olayemi O. Akinnola 1, Olatunji M. Kolawole 5, Grace I. Olasehinde 1
1Department of Biological Sciences, Covenant University, Ota. Ogun State, Nigeria
2Department of Research and Evaluation, Datametrics Associates Limited, Abuja, Nigeria
3Datanet Strategy and Analytics Consulting Limited, Abuja, Nigeria
4Department of Microbiology, Faculty of Life Sciences, University of Ilorin, Ilorin, Kwara State, Nigeria
5Department of Epidemiology and Medical Statistics, College of Medicine, University of Ibadan, Nigeria
Correspondence to: Abiodun F. Ipadeola , Department of Biological Sciences, Covenant University, Ota. Ogun State, Nigeria.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

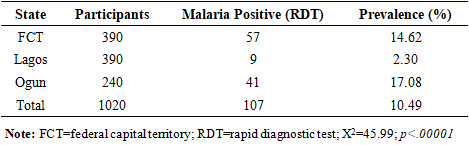
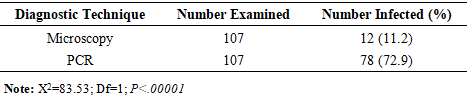
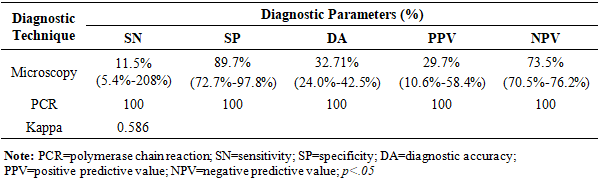
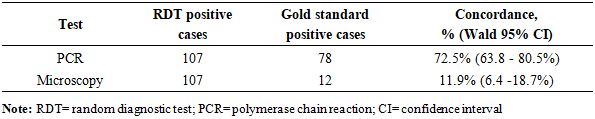
BACKGROUND: Malaria remains a critical public health challenge in Nigeria, particularly in states with high transmission rates. Accurate and timely diagnosis is essential for effective treatment and control, yet the comparative performance of available diagnostic tools varies widely in resource-limited settings. AIM: This study aims to evaluate and compare the diagnostic accuracy of microscopy, polymerase chain reaction (PCR), and malaria rapid diagnostic test (mRDT) in detecting malaria cases in high-burden states in Nigeria. It seeks to provide evidence for optimizing diagnostic strategies and strengthening malaria control programs in resource-limited settings. METHODS: A hospital-based study was conducted in three states from October 2022 to September 2023. Suspected malaria cases were referred for testing using SD-Bioline™ mRDT, and positive samples confirmed using microscopy and nested Polymerase Chain Reaction (nPCR). Diagnostic accuracy and Kappa statistics were calculated. RESULTS: Out of 107 mRDT-positive samples, further analysis was conducted using both microscopy and nested PCR (nPCR). PCR confirmed 72.9% (78 samples) as positives, whereas only 11.2% (12 samples) (p<0.00001) were positive by microscopy. The sensitivity and specificity of microscopy compared to PCR were 11.5% (95% CI: 5.4 –20.8%) and 89.7% (95% CI: 72.7–97.8%), respectively, with a Kappa index of 0.586 (p<0.05). The concordance rates for PCR and microscopy with mRDT were 72.5% (95% CI: 63.8–80.5%) and 11.9% (95% CI: 6.4 –18.7%), respectively. CONCLUSION: The study reveals inherent challenges with the use of microscopy in diagnosing malaria in Nigeria. However, the high concordance between mRDT and PCR outcomes underscores the reliability of mRDTs for malaria diagnosis, facilitating prompt treatment and potentially reducing malaria morbidity and mortality.
Keywords: Malaria Rapid Diagnosis, Microscopy, Nested Polymerase Chain Reaction
Cite this paper: Abiodun F. Ipadeola , Elvis E. Isere , Lazarus O. Omenyi , Timothy A. Attah , Oladipupo B. Ipadeola , Olayemi O. Akinnola , Olatunji M. Kolawole , Grace I. Olasehinde , Diagnostic Performance of Rapid Diagnostic Test, Microscopy, and Polymerase Chain Reaction for Malaria Diagnosis in High-burden States in Nigeria, Public Health Research, Vol. 15 No. 2, 2025, pp. 57-64. doi: 10.5923/j.phr.20251502.03.
Article Outline
1. Introduction
- Malaria has been a major global health challenge for centuries, particularly in endemic regions where tropical climates support the breeding of the female Anopheles mosquito, the primary vector of the disease [1,2]. Malaria is the most prevalent acute febrile illness (AFI) in sub-Saharan Africa [3-5]. Sub-Saharan Africa accounts for about 94% of malaria cases and 95% of related mortalities globally, highlighting the persistent burden despite significant progress in malaria control in these countries [1]. The World Health Organization (WHO) also estimates that Nigeria contributes to about 26% of the global malaria burden in 2023, with high morbidity and mortality rates, especially among children under five years old and pregnant women [6]. Despite a 27% global decline in malaria incidence between 2000 and 2015, progress plateaued thereafter, with a decline of less than 2% decline by 2019 and a subsequent increase of 2% by 2023 compared to 2015 levels, indicating limited progress in alleviating the burden of malaria infections in recent years [6,7]. Similarly, in Nigeria, malaria incidence declined by 18%, from an estimated 262 million cases in the year 2000 to 214 million in 2015, and further to 66.7 million in 2023, reflecting substantial national progress in reducing malaria burden [8,9].In Nigeria, the risk of transmission still exists across the country and persists all year-round, with about 97% at risk and 76% of the population living in high-transmission areas [10]. While the incidence of malaria continues to decline across the country, Kano, Lagos, and the Federal Capital Territory (FCT) have seen a significant increase in malaria cases [11]. Given that prompt treatment, control, management, and elimination of malaria critically depend on early and accurate detection of malaria parasites in infected individuals, the rising challenge of diagnostic inaccuracies attributable to varying sensitivities across available diagnostic tools [12-14] serves as a key motivation for this study. Accurate identification of the causative Plasmodium species, along with assessing parasitemia levels and associated clinical and biochemical markers, is crucial for guiding appropriate malaria management [5]. Microscopic examination of thick and thin blood films stained with Giemsa or May-Grunwald-Giemsa remains the gold standard for malaria diagnosis, as it enables parasite detection, species identification, quantification of parasitemia, and differentiation between sexual and asexual parasite forms [5]. However, the efficacy of microscopy is highly dependent on the skill and experience of the Laboratory personnel, the quality of the reagents, and the equipment used, all of which can affect the sensitivity and specificity of the diagnostic method [15,16]. The method is also labor-intensive, time-consuming, and requires extensive training, which poses a challenge in many African countries where the capacity for high-quality malaria microscopy remains relatively low [17]. Real-time polymerase chain reaction (PCR) is considered the first-line assay to confirm malaria diagnosis by microscopy, given its ability to detect and amplify Plasmodium DNA and identify low-level parasitemia and mixed-species infections [12], [18]. To ensure proper and rapid malaria diagnosis for effective treatment, the WHO recommends confirmatory parasitological diagnosis using microscopy, alongside malaria rapid diagnostic tests (mRDTs) for point-of-care diagnosis before administering antimalarial treatment [19]. MRDT is an antigen-based tests that detect specific Plasmodium proteins, such as histidine-rich protein 2 (HRP2), Plasmodium lactate dehydrogenase (pLDH), and aldolase, in a small blood sample, providing results within 15–20 minutes [20]. HRP2 is specific for detecting Plasmodium falciparum, while pLDH and aldolase can detect all four human-infecting Plasmodium species but are unable to differentiate between them [21]. Microscopy and malaria histidine-rich protein-2 RDTs have been integrated into Nigeria’s national malaria treatment guidelines as part of the country’s malaria control policy and have been found to accurately detect and differentiate P. falciparum from other species [17], [22]. A systematic review of studies conducted primarily in Europe evaluating fourteen different rapid diagnostic tests (RDTs) for P. falciparum and P. vivax, using microscopy and polymerase chain reaction (PCR) as reference standards, reported a sensitivity ranging from 67.9–100% and specificity of 93.1–100% for P. falciparum. For P. vivax, sensitivity ranged from 66–91%, while specificity was between 98–100% [23]. Another study on 330 participants found that 18S nested PCR detected malaria in 24.55% of participants, while microscopy and RDTs identified 10.91% and 9.4% of cases [24]. Using PCR as the gold standard, microscopy failed to detect 59.3% of malaria cases, while RDTs missed 63%, demonstrating their lower sensitivity in malaria detection [24]. Numerous other studies have found to an extent, that microscopy has low performance for the detection of submicroscopic malaria infections when compared to PCR as a gold standard [12], [14], [25].While PCR is the most sensitive in detecting malaria parasites and is usually considered the gold standard, this procedure is scarcely available, especially in resource-limited areas like Nigeria. As a result, less cost-intensive methods such as microscopy and mRDT have been the most affordable diagnostic methods for detecting malaria in Nigeria. Understanding the comparative performance of these diagnostic tools in detecting malaria is crucial for enhancing diagnostic accuracy, ensuring prompt treatment, and ultimately reducing the negative outcomes due to malaria infection. This study aims to comparatively analyze the diagnostic accuracy of microscopy, PCR, and other rapid diagnostic tools in detecting malaria in high-transmission states of Nigeria.
2. Methods
- This section details the methodological framework employed in the study, encompassing the research design, participant recruitment, diagnostic procedures, and statistical analyses used to evaluate malaria diagnostic tools.
2.1. Study Design
- A hospital-based cross-sectional study was conducted on 1,020 patients suspected of malaria in health facilities in the Federal Capital Territory (FCT), Lagos, and Ogun states between October 2022 and September 2023.
2.2. Eligibility Criteria
- Any case patient presenting with signs and symptoms of malaria (such as fever, headache, malaise, and nausea, among other symptoms) referred by clinicians to the laboratory for malaria diagnostic tests in selected hospitals, who voluntarily consented to participate in the study, were included. Written informed consent was obtained from participants aged 18 years and older. However, parental or guardian consent was obtained from the parents of those under the age of 18 years. Patients with no clinical symptoms related to malaria and potential participants who were unable to provide informed consent were excluded from the study.
2.3. Sampling and Sample Size
- The FCT, Ogun, and Lagos states were selected based on their epidemiologic relevance [3]. The sample size was determined using a 5% margin of error at a 95% confidence level (α = 0.05, power 1 – β = 0.20) and an estimated 26.8% malaria prevalence [15], [16], [26], [27]. The final sample size calculation indicated that a minimum of 301 participants was required for the study. All eligible participants were recruited, and consenting participants were included until the sample size was achieved.
2.4. Laboratory Investigation
- Capillary blood was collected from the finger tip for mRDT testing, and 2 ml of venous blood was collected from each participant into EDTA tubes for microscopy and nested PCR (nPCR) testing. Preliminary screening using mRDT was performed immediately using SD-Bioline™ RDT (Standard Diagnostic Inc., Korea), and this was performed by trained hospital laboratory scientists at each study site. Blood samples were also collected for microscopy and PCR diagnosis.
2.5. Microscopy Diagnosis
- Thick and thin blood films were prepared in duplicate for each participant on clean, well-labelled, frosted glass slides. For this, 2µL and 6µL of whole blood were pipetted for thin and thick films, respectively [15], [16], [28], [29]. The thin smears were fixed in absolute methanol, and the slides were arranged in slide boxes for further analysis. All slides were stained using 10% Giemsa working solution and subsequently imaged at the ×100 objective. Parasite detection involved examining at least 100 high-power fields [29]. Microscopy diagnosis was conducted by two independent malaria microscopists.
2.6. PCR Diagnosis
- Extracted DNA from dry blood spot samples was screened for the presence of P. falciparum. Primers (rPLU5 and rPLU6 of P. falciparum species) were used for nested PCR of the 18S rRNA gene in malaria parasites [3], [30], [31], [32]. These primers include rPLUS5 CCTGTTGTTGCCTTAAACTTC and rPLU6 TTAAAATTGTTGCAGTTAAAACG, and were sequenced to convert P. falciparum species 18S rRNA gene to complementary DNA (cDNA) [30,31].A step-by-step working protocol for the PCR reaction was designed, and all reagents as well as primers (purchased from manufacturers) were reconstituted to the working protocol specification (concentrations and volumes) using the formula below;
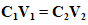 Where C1 = Initial concentrationV1= Initial volumeC2 = Final concentrationV2 = Final volumeFor the nested PCR, the first amplification reaction used was 2μl of individual gDNA in a 20μl reaction mixture (0.25 mM each dNTP, 10 mM Tris-HCl [pH 9.0], 30mM KCl, 1.5mM MgCl2, and 1.0 units of Taq polymerase) containing 0.02μM primers (P1_F and P2_R) [3], [33], [34]. The DNA amplification was conducted using the cycling condition as instructed by the manufacturer (Table 4 in Appendix) under a 95°C temperature for 5 min and then 35 cycles at 95°C for 30 s, 58°C for 1.5 min, and 72°C for 1 min, followed by a final extension at 72°C for 5 min [3], [35]. The first PCR product was diluted 20-fold in sterile water. Just 1μl of this solution was used in the second amplification. The reactions were performed in four steps: initial denaturation at 95°C for 5 min; denaturation at 95°C for 30 seconds; annealing at 60°C for 1 min; and extension at 72°C for 30 seconds [3], [35], [36].The second and fourth steps were repeated 20 times, and step 4 was performed for 10 minutes with the P1_F forward primer in combination with the reverse primer. The amplified products were visualized in 2% agarose gels stained with ethidium bromide [37].
Where C1 = Initial concentrationV1= Initial volumeC2 = Final concentrationV2 = Final volumeFor the nested PCR, the first amplification reaction used was 2μl of individual gDNA in a 20μl reaction mixture (0.25 mM each dNTP, 10 mM Tris-HCl [pH 9.0], 30mM KCl, 1.5mM MgCl2, and 1.0 units of Taq polymerase) containing 0.02μM primers (P1_F and P2_R) [3], [33], [34]. The DNA amplification was conducted using the cycling condition as instructed by the manufacturer (Table 4 in Appendix) under a 95°C temperature for 5 min and then 35 cycles at 95°C for 30 s, 58°C for 1.5 min, and 72°C for 1 min, followed by a final extension at 72°C for 5 min [3], [35]. The first PCR product was diluted 20-fold in sterile water. Just 1μl of this solution was used in the second amplification. The reactions were performed in four steps: initial denaturation at 95°C for 5 min; denaturation at 95°C for 30 seconds; annealing at 60°C for 1 min; and extension at 72°C for 30 seconds [3], [35], [36].The second and fourth steps were repeated 20 times, and step 4 was performed for 10 minutes with the P1_F forward primer in combination with the reverse primer. The amplified products were visualized in 2% agarose gels stained with ethidium bromide [37].2.7. Study Variables
- Sensitivity: Using PCR as the standard test, sensitivity was calculated as the number of specimens identified as positive by the microscopy test divided by the number of specimens identified as positive by the PCR test and expressed as a percentage [38-40].Specificity: Using PCR as the standard test, specificity was calculated as the number of specimens identified as negative by the microscopy test divided by the number of specimens identified as negative by the PCR test and expressed as a percentage [38-40].Positive Predictive Value: This is defined as the ratio of individuals correctly diagnosed as positive compared to all positive test results [38-40].Negative Predictive Value: This refers to the ratio of individuals correctly diagnosed as negative cases compared to all negative test results [38-40].
2.8. Statistical Analysis
- All test results were collected in a Microsoft Excel version 365 spreadsheet for data cleaning and coding. MedCalc was used to compare the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of microscopy compared to nPCR. Agreement between pairs of diagnostic methods was determined using Cohen’s Kappa statistics, with values ≤0 indicating no agreement, 0.01−0.40 slight to average agreement, 0.41−0.80 moderate to substantial agreement, and 0.81−1.00 indicating perfect agreement, while the level of significance was set at a p-value<0.05. The concordance between mRDT and nPCR test outcomes, as well as between mRDT and microscopy results, was assessed by calculating the proportion of mRDT-positive samples that were confirmed by nPCR or microscopy, respectively. This was computed using the “Measuring U” tool, which also provided the associated Wald 95% confidence intervals (CIs).
2.9. Ethical Approval
- Ethical approval was obtained from the Ogun State Research Ethics Committee with protocol approval number HPRS/381/449, the Lagos State Research Ethics Committee at the Lagos State University Teaching Hospital (LASUTH) with protocol approval number LREC/06/10/1815, and the FCT-Abuja Health Research Ethics Committee with protocol approval number: FHREC/2022/01/174/05-09-22. Participants for the study were recruited voluntarily after providing informed consent. Also, written informed consent was obtained from the respondents. All the information obtained from participants is confidential and archived in a secure database only accessible to the authors of the study.
3. Results
- A total of 1,020 individuals suspected of malaria were recruited for the study, comprising 390 participants each from the Federal Capital Territory (FCT) and Lagos State, and 240 from Ogun State. Out of the 1,020 individuals screened using mRDT, 913 (89.5%) tested negative and were excluded from further analysis. The remaining 107 participants (10.5%) tested positive and were subsequently evaluated using microscopy and PCR. Malaria prevalence based on mRDT results was notably higher in Ogun State (17.08%) and the FCT (14.62%) compared to Lagos State (2.30%), with X2=45.99; and p<.00001. The analysis of the participant enrollment is provided in Table 1.
|
|
|
|
4. Discussion
- This study found that out of 1,020 febrile cases, the prevalence of malaria by mRDT was 10.49%. The prevalence of malaria was higher in FCT and Ogun State compared to Lagos State. However, the prevalence of malaria in this study was much lower compared to previous studies conducted in other parts of the country [11], [41], [42]. While numerous studies have shown a higher prevalence of malaria across the different Nigerian populations, a possible reason for the lower prevalence based on RDT diagnosis could be as a result of seasonal differences, lower sensitivities and specificities, or inadequate use of RDT.We also observed that the sensitivity of microscopy in our study was much lower compared to other studies [12], [25], [43]. However, these studies also reported sensitivities of <60%, indicating the possibility of microscopy missing out a significant proportion of true malaria cases. Our finding is also in resonance with another study, which reported a very low diagnostic sensitivity of 8.9% for microscopy in the detection of malaria in the southwestern states of Nigeria [12]. Low sensitivity of microscopy could also be a result of reliance on visualization of the parasite, which is largely dependent on the skills and experience of the microscopist and is prone to human errors [43]. Low sensitivity of microscopy in the diagnosis of malaria can also be attributed to low parasite density [44]. Low-level parasitemia is common in hyperendemic areas and is usually observed in individuals with adequate host immunity or those who have received antimalarial treatment [44]. PCR offers high sensitivity as seen in our study, and is capable of detecting fewer parasites per microliter of blood. However, this diagnostic technique is relatively expensive, time-consuming, and requires high-level expertise [43].Our study found a relatively high specificity of microscopy, which is consistent with other studies [43], [45]. The high specificity of microscopy in detecting malaria makes it a good diagnostic technique for identifying non-malaria cases and minimizing the risk of detecting false positive cases. This is mostly crucial in countries like Nigeria, where there is a burden of other febrile illnesses like viral hemorrhagic fevers and arboviral infections. Thus, this diagnostic tool can be effective in preventing misdiagnosis and mistreatment of febrile cases. However, this prospect can be hampered by low sensitivity, which can affect the potential to effectively detect true cases, especially cases with low parasitemia.One important finding from our study is the high concordance between mRDT and the PCR tests, with an overall concordance of 72.5%, demonstrating mRDTs as a reliable tool for malaria diagnosis in Nigeria. Given the accessibility, cost-effectiveness, and simplicity of this tool [22], mRDTs are particularly valuable in low- and middle-income countries (LMICs) like Nigeria, where there are limited resources and trained microscopists to carry out diagnosis using more advanced tools [22], [46], [47], [48], [49]. MRDTs can detect more than 100 parasites per microliter (0.002% parasitemia) and provide results quickly, typically within 15 to 20 minutes [43], [47]. These tests are available commercially as kits and are easy to perform, requiring minimal training, equipment, or complexity in result interpretation [43], [47]. However, one primary limitation of mRDTs lies in their specificity, as antigens can remain in the patient's blood after treatment, leading to false-positive results [43], [47].While microscopy is considered the gold standard for malaria diagnosis due to its cost-effectiveness compared to other laboratory methods, it suffers from its low sensitivity, which makes it difficult to detect true positive cases. Accurate results depend on well-trained microscopists, and in their absence, reproducibility of diagnosis may decline, sensitivity may vary, and false-positive rates can become unacceptably high. Misdiagnosis of malaria may affect timely treatment and the continued transmission of malaria across the population. Also, this could result in underreporting, which undermines accurate data collection and effective public health actions to mitigate the burden of malaria in Nigeria. In contrast, mRDT remains the most accessible and cost-effective diagnostic tool for malaria diagnosis in many low- and middle-income countries (LMIC) like Nigeria. Hence, mRDTs are preferred options for timely diagnosis, treatment, and programmatic deployment in Nigeria.
4.1. Study Limitations
- There were some limitations identified in this study. First, this study adopted a cross-sectional design and does not capture long-term trends. Second, the exclusion of mRDT-negative patients who may have been diagnosed by PCR. Third, while all PCR analyses were conducted in a centralized, quality-assured laboratory, sample transport logistics posed risks to degradation, which could affect diagnostic accuracy. However, these risks were mitigated through the use of validated cold-chain protocols and strict adherence to standardized operating procedures during sample handling and processing.
5. Conclusions
- This study highlights critical diagnostic gaps in malaria detection within Nigerian healthcare facilities, particularly the limited sensitivity of microscopy, which may hinder timely and accurate case identification. While microscopy remains a cost-effective tool with high specificity, its reliance on operator skill and vulnerability to low parasite densities reduces its utility as a standalone diagnostic method. In contrast, mRDTs demonstrated high concordance with PCR and offer a practical, accessible, and rapid alternative, especially in resource-constrained settings. Given these findings, strengthening malaria diagnostic strategies through the routine integration of mRDTs, supported by PCR confirmation in complex or ambiguous cases, could significantly improve case detection, guide appropriate treatment, and support malaria elimination efforts in Nigeria and other endemic regions.
ACKNOWLEDGEMENTS
- The authors sincerely appreciate all study participants who took part in this study, as well as all research assistants and officials of each of the health institutions for their support during the data collection stage of the study.
FUNDING
- No funding was received for this study.
DATA AVAILABILITY
- The data sets used or analyzed during this study are available from the corresponding authors upon reasonable request.
AUTHORS’ CONTRIBUTION
- Authors AFI conceptualized the study and devised the methodology, including data collection. Authors AFI, EEI, and OBI curated the data and performed the formal analysis. AFI, EEI, OBI, and PEO wrote the original draft of the manuscript. OOA, OMK, and GIO supervised the study. All authors reviewed and edited the paper. All the authors have read and approved the paper.
DISCLAIMER
- The authors alone are responsible for the views expressed in this article, and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated.
CONFLICT OF INTEREST
- The authors declare that there is no conflict of interest.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML