-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Public Health Research
p-ISSN: 2167-7263 e-ISSN: 2167-7247
2024; 14(1): 12-21
doi:10.5923/j.phr.20241401.02
Received: Jan. 14, 2024; Accepted: Jan. 30, 2024; Published: Feb. 5, 2024

Budget Impact Analysis of Cervical Cancer Treatment in Kenya
Easter Olwanda 1, Elizabeth Owiti 1, Njuguna David 2
1School of Economics, University of Nairobi, Nairobi, Kenya
2Department of Central Planning and Project Monitoring, Ministry of Health, Nairobi, Kenya
Correspondence to: Easter Olwanda , School of Economics, University of Nairobi, Nairobi, Kenya.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

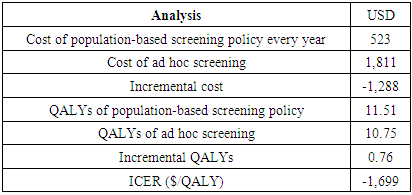
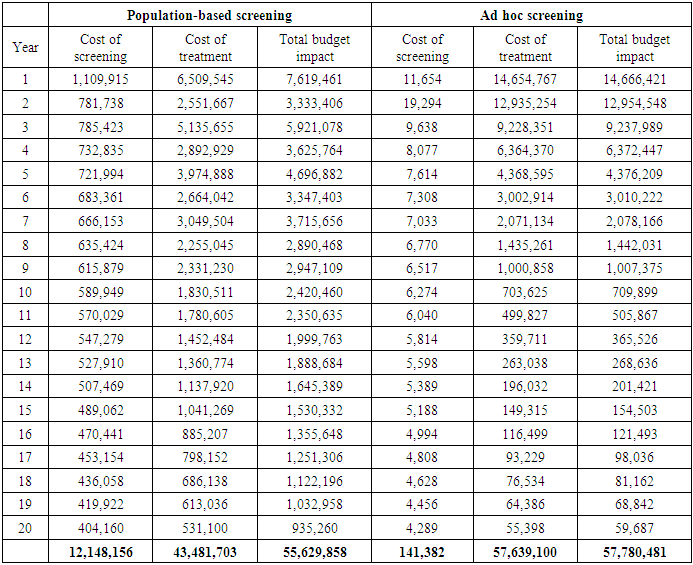
Cervical cancer continues to impose a significant burden on women's health globally, particularly in low- and middle-income countries such as Kenya. Inadequate screening and treatment are major contributors to the high incidence and mortality rates associated with this disease. This study aimed to model the budget impact of screening and treatment in Kenya. To achieve this objective, a budget impact model was developed. The incremental costs for treating these individuals were obtained from the output of the Markov model. We employed a health system perspective, focusing exclusively on the direct medical costs borne by the provider. To establish the input parameters, we drew information from various sources. The cost data were derived from the One Health Model. The first policy option examined in this study involved a population-based Cervical Cancer (CC) screening program, which was evaluated alongside the ad hoc screening (also referred to as screening at the point of care) using a decision tree The model projected the budget impact over a 20-cycle period, considering the coverage of 100,000 women. The model also factored in a discounting rate of 3%, which is utilized to adjust the value of future outcomes in economic evaluations. The annual cost of the population-based screening policy is $523, while the cost of ad hoc screening is higher at $1,811. The incremental cost, which represents the additional cost of implementing the population-based screening policy compared to ad hoc screening, is -$1,288, indicating potential cost savings. In terms of QALYs, the population-based screening policy yields 11.51 QALYs, while ad hoc screening results in 10.75 QALYs. The incremental QALYs, representing the additional health benefits of the population-based screening policy, are 0.76. The incremental cost-effectiveness ratio (ICER) is -$1,699 per QALY, suggesting that the population-based screening policy is cost-effective compared to ad hoc screening Over a period of 20 years, the cost of screening decreases to $404,160, with the cost of treatment amounting to $531,100. The total budget impact for this approach over 20 years is projected to be $935,260. On the other hand, the ad hoc screening approach has significantly lower costs. In the first year, the cost of screening is estimated to be $11,654, with the cost of treatment being $14,654,767. This results in a total budget impact of $14,666,421. Similarly, over a period of 20 years, the cost of screening decreases to $4,289, while the cost of treatment decreases to $55,398. The total budget impact for ad hoc screening over 20 years is projected to be $59,687. Finally, the conclusion drawn from the study is that implementing a population-based screening policy can lead to significant cost savings for healthcare systems. This policy eliminates the need for individual screening decisions, resulting in reduced administrative burden and costs. By implementing a systematic screening program based on predetermined guidelines, all individuals in the population can be effectively screened. This standardized approach optimizes resource utilization, reduces unnecessary testing, and alleviates financial burdens for both patients and the healthcare system.
Keywords: Cervical Cancer, Budget Impact Analysis, Population-based screening and treatment, Ad-hoc Screening and treatment, Costs, Model, Decision tree
Cite this paper: Easter Olwanda , Elizabeth Owiti , Njuguna David , Budget Impact Analysis of Cervical Cancer Treatment in Kenya, Public Health Research, Vol. 14 No. 1, 2024, pp. 12-21. doi: 10.5923/j.phr.20241401.02.
1. Introduction
- Cervical cancer ranks fourth among cancers affecting women globally, with an incidence of 570,000 in 2018 and above 311,000 mortalities reported annually. More than 85% of these occur in low and middle-income countries (LMICs) (Bray et al. 2018). By 2030, thirty million people will die from different types of cancers annually, and three-quarters of these deaths will occur in LMICs if no significant investments in prevention are made (GLOBOCAN 2018). Globally, the sub-Saharan region, South America, and Latin America have the highest cervical cancer incidence. The age-standardized incidence was 31 out of every 100,000 women in sub-Saharan Africa (Louie, De Sanjose, and Mayaud 2009). North America had a low age-standardized incidence of 7 among every 100,000 women. The age-standardized mortality attributed to cervical cancer in Africa was 23 out of 100,000 annually (Ferlay et al. 2015). Cervical cancer is one of the most common cancers among women in Kenya, contributing to a substantial portion of cancer-related morbidity and mortality. In 2017 Kenya reported an age-standardized incidence rate of 40.1 per 100,000 women (ICO 2017). Late-stage diagnosis is common, leading to lower survival rates. Lack of awareness, limited access to healthcare services, and cultural factors contribute to delayed or inadequate screening and treatment (Tekalign and Teshome 2022). The prevalence of high-risk human papillomavirus (HPV) infections, a major cause of cervical cancer, is relatively high. Cervical cancer affects women's physical and emotional well-being, often resulting in significant pain and suffering (Kwete et al. 2023). Late-stage cases may require more aggressive and costly treatments, impacting the overall quality of life. The economic burden of cervical cancer includes direct medical costs for treatment, as well as indirect costs related to lost productivity, caregiver burden, and the strain on healthcare systems. Families may face financial challenges due to the costs associated with cancer care (Ngcamphalala, Östensson, and Ginindza 2021).Cervical cancer can be prevented through vaccination, screening, and treatment of pre-cancers. However, there is a disproportionately high number of new cases and deaths in third-world settings that do not have structured prevention strategies (Pimple and Mishra 2019). Dealing with disparities in the burden of cervical cancer disease is one of the post-2015 global development agenda. The WHO recommends strategies tailored around the female lifecycle using the natural history of the illness. The WHO position paper proposes that countries with a high cervical cancer incidence incorporate cost-effective, sustainable, and feasible HPV vaccination in countrywide plans for girls between 9-14 years, before being exposed to or acquiring HPV infection. It also approves the implementation of economical or easy-to-use screening techniques. Moreover, it endorses tertiary care to treat invasive cancer through radiotherapy, chemotherapy, and ablative surgery (World Health Organization 2013).Limited access to cervical cancer screening services, especially in rural areas, contributes to late-stage diagnoses (Zahnd et al. 2018). Education and awareness programs are crucial to encouraging women to seek regular screenings. Inadequate healthcare infrastructure, including a shortage of trained healthcare professionals and limited resources, can hinder the delivery of timely and effective cervical cancer care (Maseko, Chirwa, and Muula 2015). The implementation of preventive measures, such as HPV vaccination and improved screening programs, faces challenges due to resource constraints and logistical issues. The Kenyan government, along with international organizations and NGOs, has been working to address the burden of cervical cancer through awareness campaigns, vaccination programs, and initiatives to improve screening and treatment services (Mwenda et al. 2022).As these effective cervical cancer prevention and treatment strategies have become available, resource constraints and cost concerns have increasingly been at the center of the debate. Kenya faces an enormous economic burden associated with cervical cancer including healthcare costs and productivity losses. Moreover, the health system in Kenya is overwhelmed by competing healthcare needs including, the emergence of the recent COVID-19 pandemic. This highlights the lack of preparedness, structural shortcomings, and the importance of strengthening health systems by building their resilience against shock. Determining the cost of cervical cancer prevention and treatment is essential for health systems strengthening. These cost estimates are useful in agenda setting by informing equitable and efficient allocation of resources. They can be used to plan and budget, mobilize, and set user fees, set insurance premiums, and contract prices.Sinanovic et al. (2009) conducted a study in South Africa to determine the costs of a school-based vaccination program. They used an ingredients-based approach and factored in costs for the HPV vaccine for three doses ($360), a booster administration ($120), four administration visits, and estimated 15% wastage costs. They also assumed the booster administration would occur in clinics and added 25% to program costs, resulting in an estimated overall vaccination program cost of $570 (Sinanovic et al. 2009). In Mozambique, a micro-costing approach was used to calculate both the financial and economic costs of vaccination. The economic cost for a single dose was US$17.59, and full immunization per girl cost US$52.29. In contrast, the financial cost for a single dose was US$6.07, compared to US$17.95 for full immunization. However, when the number of doses and personnel were reduced, the economic cost for a single dose and full immunization was US$15.53 and US$31.14, respectively (Alonso et al. 2019).In a recent study, the cost of cervical cancer screening was estimated for provider collection and self-collection using careHPV in three countries. The direct medical cost for careHPV through provider collection was higher, at 9.24, 15.61, and 8.78 for India, Nicaragua, and Uganda, respectively, compared to clinic-based self-collection, which cost 8.90, 13.48, and 8.48 for India, Nicaragua, and Uganda, respectively (Campos, Tsu, et al. 2017). Estimates similar to that of self-collection in Nicaragua were reported by Mezei et al (2018) who reported the direct medical costs for the Self-collected HPV test at US$12.73 per woman (Mezei et al. 2018). In contrast, the cost of self-collection and provider collection were comparable in rural Shanxi Province, China. The estimated costs were US$7.49 and $7.95 respectively (Shi et al. 2012). In Kenya, screening at community health campaigns and clinics costs $25.00 and $29.56 per woman respectively (Shen et al. 2018). HPV DNA costs have also been estimated from a societal perspective. Zimmermann et al (2017) reported the screening costs for women with a low CD4 count of between 200 and 500 cells/mL at $223 and $113 from a societal and clinic perspective respectively. Moreover, HPV DNA cost women for with higher CD4 counts (>500 cells/mL) is $102 from a societal perspective and $56 from a clinic perspective (14). Contrary to this finding, Sinanovic et al (2009) reported the societal cost per for HPV DNA test at $309 per woman (Sinanovic et al. 2009).Several studies have estimated the costs of cryotherapy. Mvundura & Tsu (2014) focused on estimating the costs of VIA coupled with cryotherapy for both a single-visit approach and a two-visit approach. They found that the economic cost of receiving cryotherapy at health centers through a single-visit scenario was US $70.91, while the cost at regional hospitals within a two-visit scenario was US $37.58. These findings align closely with those of Quentin et al. (2011), who estimated the incremental costs of cryotherapy for a single-visit scenario in Ghana and reported a range of US$ 47.26 to US$ 84.48 at the surveyed facilities (Quentin et al. 2011). Campos et al (2015) estimated the direct medical costs of cryotherapy and reported much lower costs at US$ 21.53 (Campos et al. 2015). Similarly, they reported lower cryotherapy costs for a two-visit scenario in Nicaragua at US$ 18.16 (Campos, Mvundura, et al. 2017) and US$ 13.49 in Uganda (Campos, Tsu, et al. 2017). In Kenya, Zimmermann et al (2017) estimated costs of universal, prophylactic cryotherapy without prior cervical cancer screening and reported US$99 and US$19 from a societal and provider perspective, respectively (Zimmermann et al. 2017). Finally, Mezei et al estimated the direct medical costs for cryotherapy treatment and reported US$5.85 per woman (Mezei et al. 2018).The available literature emphasizes the costs associated with various prevention and treatment methods. Nevertheless, there is a gap in the literature regarding the budget impact of cervical cancer screening and treatment. Budget Impact Analysis (BIA) is a type of economic evaluation used in the field of healthcare to assess the potential financial implications of adopting a new medical intervention, treatment, or technology within a healthcare system. It focuses on estimating the direct costs associated with implementing the intervention and how it might affect the overall budget of a healthcare organization, government agency, or payer. The main objective of a Budget Impact Analysis is to provide decision-makers with a clear understanding of the potential financial impact of introducing a new intervention into the healthcare system. This information can help stakeholders make informed decisions about resource allocation, reimbursement strategies, and budget planning. Consequently, this study aimed to estimate the budget impact of cervical cancer treatment in Kenya.
2. Methods
- Budget impact analysis was done to estimate the number of women needed to screen and treat cervical cancer and the annual screening and treating costs. The incremental costs for treating these individuals were obtained from the output of the Markov model. We employed a "health system perspective," focusing exclusively on the direct medical costs borne by the provider. To establish the input parameters, we drew information from various sources. The cost data were derived from the One Health Model. The first policy option examined in this study involved a population-based Cervical Cancer (CC) screening program, which was evaluated alongside the "ad hoc screening" (also referred to as screening at the point of care) using a decision tree. The decision tree enabled us to compare the costs and outcomes associated with both policies. See Figure 1 below. The second policy option is considered an annual population-based CC screening policy. To assess the costs and outcomes over a 20-year time horizon, we developed a Markov model.
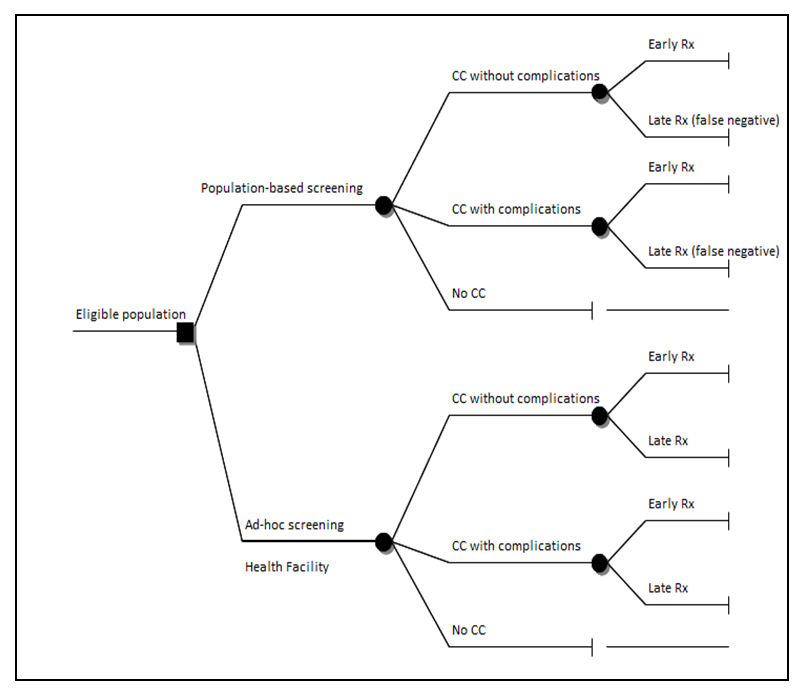 | Figure 1. Decision Tree |
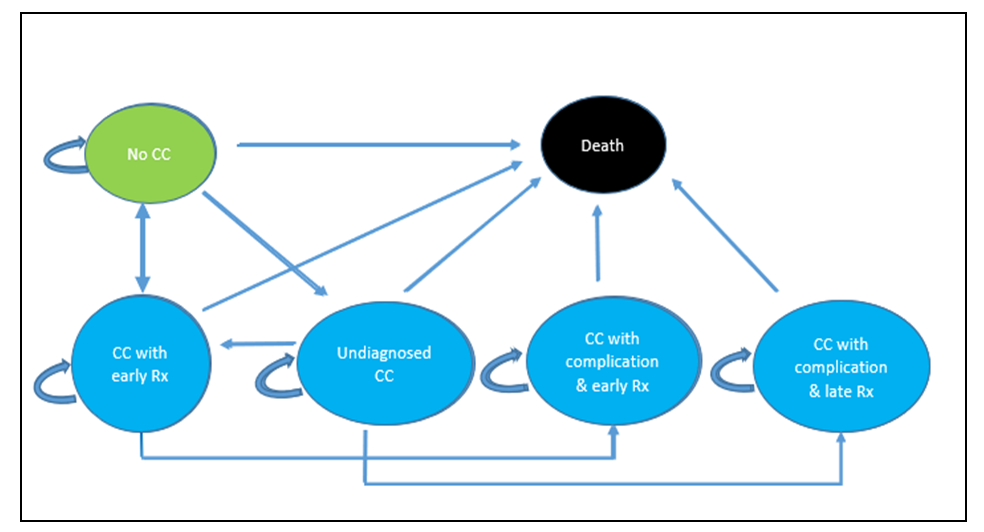 | Figure 2. Markov Model |
3. Results
- A Budget Impact Analysis (BIA) is an economic evaluation tool used to assess the financial implications of implementing a new healthcare intervention or policy within a specific budgetary framework. It offers decision-makers valuable insights into the economic consequences of adopting a particular health program or intervention, requiring a comprehensive understanding of its economic implications. When interpreting a BIA, it's important to consider the scope and perspective, which may focus on a specific healthcare system, payer, or provider, and the time horizon over which costs and outcomes are projected. Additionally, the analysis should identify various cost components, including direct and indirect costs, potential cost savings, and compare the base case scenario (without the intervention) with alternative scenarios that incorporate the intervention's costs and outcomes. Incremental costs associated with adopting the new intervention are also a key focus in BIA interpretation (Mauskopf et al. 2007).Markov Model ProjectionsThis section presents the Markov model projections comparing the costs and quality-adjusted life years (QALYs) of a population-based screening policy versus ad hoc screening. The annual cost of the population-based screening policy is $523, while the cost of ad hoc screening is higher at $1,811. The incremental cost, which represents the additional cost of implementing the population-based screening policy compared to ad hoc screening, is -$1,288, indicating potential cost savings. In terms of QALYs, the population-based screening policy yields 11.51 QALYs, while ad hoc screening results in 10.75 QALYs. The incremental QALYs, representing the additional health benefits of the population-based screening policy, are 0.76. The incremental cost-effectiveness ratio (ICER) is -$1,699 per QALY, suggesting that the population-based screening policy is cost-effective compared to ad hoc screening. These findings imply that implementing the population-based screening policy can lead to cost savings while generating additional health benefits, making it a favorable option in terms of cost-effectiveness.These findings have several implications. First, the population-based screening policy has a lower annual cost compared to ad hoc screening, indicating potential cost savings for healthcare systems or organizations implementing the policy. The incremental cost is negative, suggesting that implementing the population-based screening policy can result in cost savings of $1,288 compared to ad hoc screening. Second, the population-based screening policy yields higher QALYs (11.51) compared to ad hoc screening (10.75), indicating better health outcomes associated with the policy. The incremental QALYs of 0.76 represent additional health benefits that can be achieved by implementing the population-based screening policy. Third, the negative incremental cost-effectiveness ratio (ICER) of -$1,699 per QALY suggests that the population-based screening policy is cost-effective compared to ad hoc screening. This means that for each additional QALY gained, the policy results in cost savings rather than additional costs, see Table 1 below.
|
|
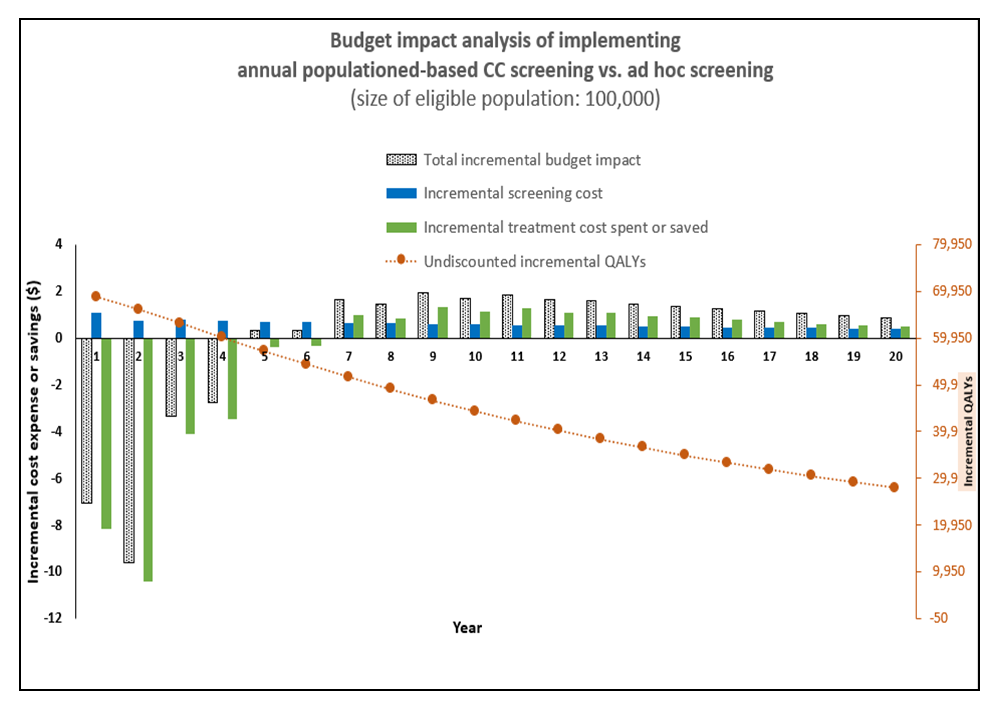 | Figure 3. Budget Impact Analysis Graph |
4. Discussion
- Efforts to reduce the burden of cervical cancer require a comprehensive and coordinated approach. This involves increasing awareness, improving access to preventive measures, strengthening healthcare infrastructure, and addressing socio-economic factors that may contribute to the challenges associated with the disease. Collaborations between the government, non-profit organizations, healthcare providers, and the community are essential for making sustainable progress in tackling the burden of cervical cancer.This study represents the budget impact analysis of cervical cancer screening and treatment in Kenya across a 20-year time horizon. Additionally, it highlights the potential opportunities to expand access to cervical cancer screening and treatment for their members while giving them more information for making coverage decisions that may expand access and minimize costs.Overall, our study findings indicate that while population-based CC screening may have significant upfront costs, the long-term benefits in terms of cost savings, improved health outcomes, and increased life years and QALYs justify the investment. These findings are consistent with those of a study done in rural Kenya that offered community-based HPV screening at pop-up tents during a two-week phase of a community health campaign. The community health campaigns had a 60% uptake compared to the control community where women were referred to their local government health facility for testing, resulting in 37% uptake (Huchko et al. 2018). In a cluster-randomized trial conducted in Argentina, a population-based approach involving Community Health Workers (CHWs) distributing HPV self-collection test kits to women at their homes yielded remarkable results. The intervention group exhibited a substantial fourfold increase in screening uptake, with an impressive 86% of women participating in the screening compared to a mere 20% in the control group (Arrossi et al. 2015). Population-based models of care shift the healthcare system from a narrow model of care targeted at the individual patient to one that focuses on the health and overall wellness of the broader population it serves (Cohen et al. 2014). Our findings demonstrate increased utilization through population-based models. This finding aligns with the benefits of integrated care as demonstrated in two settings by (Chamie et al. 2012) and (Govindasamy et al. 2013) showed increased utilization of services and identified a high burden of undiagnosed diseases through active case finding. These findings suggest that from the supply side, the opportunity to increase utilization of integrated HIV/NCD services through the population-based models of integrated care needs to be embraced broadly as this may be effective in resource-constrained settings. To combat cervical cancer mortality, it is crucial to integrate HIV/NCD prevention, diagnosis, treatment, and support. By ensuring women's access to a complete range of care and prevention measures, mortality rates can be significantly reduced. Integration efforts can involve incorporating vaccination programs, such as administering the human papillomavirus (HPV) vaccine to adolescent girls and boys, which effectively targets the primary causative virus (Karanja-Chege 2022). Furthermore, integration with screening services like Pap smears and HPV testing, as well as diagnostic services, treatment, and palliative care, can enhance the uptake of these crucial services. In addition to the aforementioned strategies, the demand side initiatives that focus on educating individuals about the risk factors associated with the disease, prevention methods, and the significance of regular screening and vaccination are critical. By disseminating accurate information and fostering a culture of knowledge, these programs empower individuals to take proactive steps toward cervical cancer prevention. Moreover, integrating health financing and insurance mechanisms is crucial to ensure that preventive measures, screening, and treatment are accessible and affordable for all, particularly for low-income populations. Efforts to combat cervical cancer involve a multi-pronged approach involving prevention, early detection, treatment, and supportive care, particularly in LMICs. Key initiatives and strategies in these regions include HPV vaccination programs targeting girls before sexual activity, expanding cervical cancer screening programs using various methods such as Pap smears, HPV testing, and visual inspections, and integrating screening services into existing healthcare programs with outreach efforts reaching women in urban and rural areas. Public awareness campaigns and educational programs aimed at both the general population and healthcare providers are also crucial, along with community engagement and mobilization of health workers to encourage women's participation in screening programs. Furthermore, efforts to strengthen healthcare infrastructure are essential to support these initiatives effectively. Implementing a population-based screening and treatment policy leads to improved health outcomes. By screening the entire population, healthcare providers can identify and address health conditions at an early stage, before they progress to more severe or irreversible states. Early detection enables timely interventions, leading to better treatment outcomes and increased chances of successful disease management. Additionally, systematic screening programs can detect asymptomatic or pre-symptomatic individuals who would otherwise go undiagnosed, allowing for early intervention and reducing the overall disease burden within the population.Efficiency in healthcare resource allocation is another key benefit of population-based screening. By implementing a comprehensive screening program, healthcare resources can be distributed more effectively and efficiently. Ad hoc screening often results in resources being allocated based on individual preferences or clinical judgment, which may lead to inconsistencies and inequities. In contrast, a population-based approach ensures that resources are allocated based on evidence-based guidelines and the needs of the entire population, promoting fairness, equity, and optimal use of resources. In summary, the findings of this study highlight the advantages of adopting a population-based screening policy over ad hoc screening. By embracing systematic screening programs that cover the entire population, healthcare systems can achieve cost savings, improved health outcomes, and more efficient allocation of resources. These implications support the implementation of comprehensive screening approaches, contributing to disease prevention, early detection, and effective management, ultimately leading to healthier populations.Our study has some limitations. We did not explicitly model changes in screening, linkage to care, or changing prevalence of cervical cancer. Another study limitation is the lack of incorporation of indirect costs in the analysis due to the absence of national data. If indirect costs were available, they may increase cost savings associated with both population-based and ad hoc cervical cancer screening and treatment. Finally, patient preferences in cervical cancer screening and treatment selections were not considered in this study.
5. Conclusions
- In conclusion, given that population-based screening has a lower cost of treatment and a lower total budget impact, it is recommended to consider implementing or expanding population-based screening programs. Advocacy efforts can be made to secure funding from government agencies, health insurance providers, or other relevant stakeholders. Demonstrating the potential cost savings and improved health outcomes associated with population-based screening can help make a case for adequate funding and reimbursement mechanisms. Moreover, by regularly assessing the program's performance, healthcare organizations can identify areas for improvement and make necessary adjustments to optimize the screening process and maximize cost savings.
Conflicts of Interest
- The authors declare that they have no conflicts of interest.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML