-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Public Health Research
p-ISSN: 2167-7263 e-ISSN: 2167-7247
2023; 13(1): 15-31
doi:10.5923/j.phr.20231301.02
Received: Feb. 15, 2023; Accepted: Mar. 25, 2023; Published: Apr. 15, 2023

Predictors of Malaria Vaccine Hesitancy Among Caregivers in Bumula Subcounty, Bungoma County
Ezekiel Chepkiyeng1, Alice Lakati1, Anselimo Makokha2
1Department of Community Health, Amref International University, Nairobi, Kenya
2Jomo Kenyatta University of Agriculture and Technology, Nairobi, Kenya
Correspondence to: Ezekiel Chepkiyeng, Department of Community Health, Amref International University, Nairobi, Kenya.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

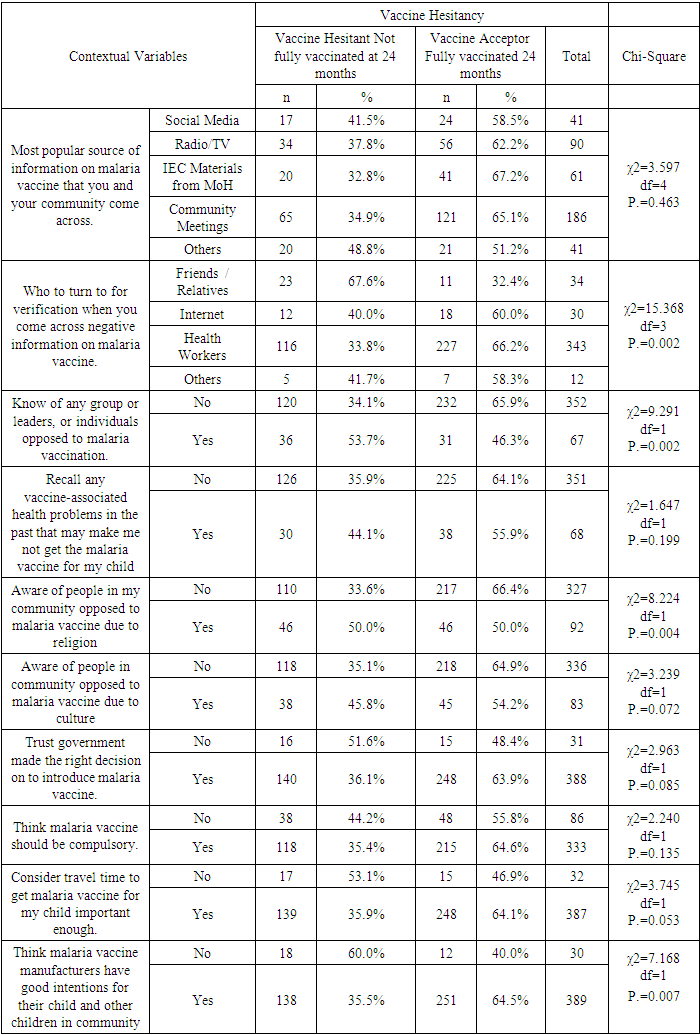
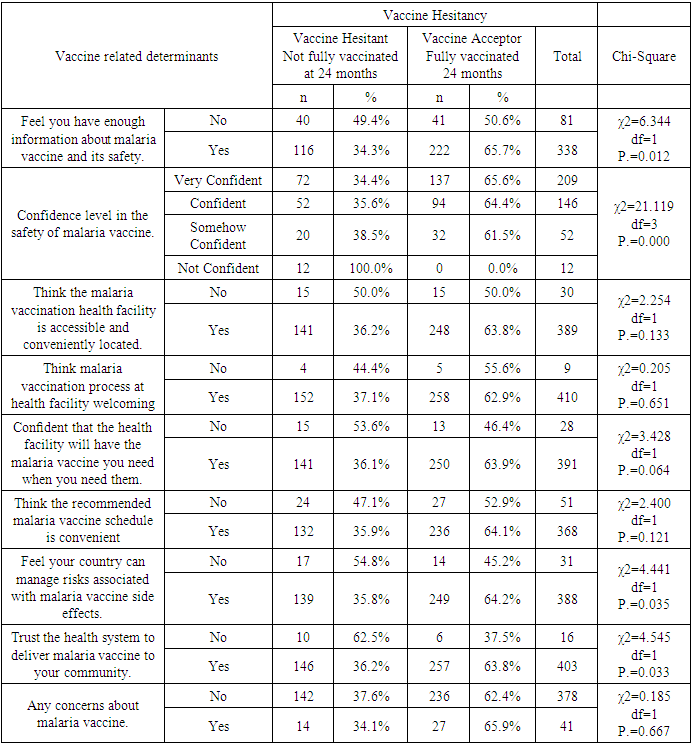
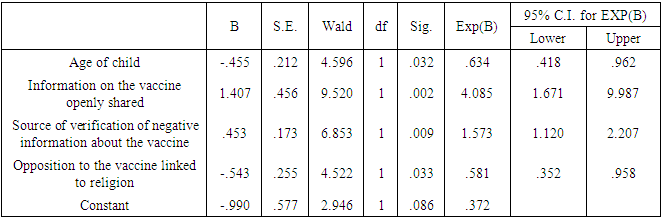
Background: Malaria is a disease that threatens health and economy of the world and is number nine among the diseases in contributing to high mortality and disability worldwide. Available WHO-recommended malaria control strategies are becoming less effective due to drug and insecticide resistance and parasite undetectability. In 2019, Mosquirix (RTS, S) vaccine was introduced to complement the existing package towards malaria prevention in children, making malaria a vaccine preventable disease (VPD). Vaccine hesitancy (VH) is a developing pattern in global health. Broad Objective: To determine the predictors of malaria vaccine hesitancy among caregivers in Bumula subcounty, Bungoma County, Kenya. Methodology: The cross-sectional study sampled 419 caregivers and their children eligible for four malaria vaccine doses by December 2022. Caregivers were interviewed face-to-face using a structured customized WHO-SAGE vaccine hesitancy questionnaire. Qualitative data was collected through 4 focus group discussions (FGDs) with 38 community health volunteers (CHVs) and key informant interviews (KIIs) with 10 key informants (KIs). Quantitative data was entered in SPSS version 28.0.1. Chi-square test was used at bivariate level and logistic regression at multivariate level. Significance level was set at 5%. Qualitative data was coded, categorized, summarized, and entered in WHO-SAGE BeSD qualitative data analysis template, where a framework was used to generate results. Findings: Out of 419 caregivers, 86.9% were female while 13.1% were male, mean age was 31.31 years and ranged from 17 to 80 years. Majority (71.8%) were married, and 89.5% Christian. Out of the 419 children, 52.5% were male and mean age was 29.32 months and ranged from 24 and 46 months. The uptake of first dose was 97.6%, which reduced to 96.2% for second dose, 86.6% for the third dose and finally 62.8% for the fourth dose. Vaccine hesitancy was at 37.2%, while vaccine acceptance was 62.8%. There were 13 significant independent variables from the chi-square bivariate analysis; religion (χ2=13.274, df=3, P.=0.004) age of child (χ2=6.739, df=2, P.=0.034), relationship between caregiver and child (χ2=13.287, df=3, P.=0.004), previous decision not to get malaria vaccine for the child (χ2=5.523, df=1, P.=0.019), feeling that information on malaria vaccine was being openly shared (χ2=12.146, df=1, P.=0.00), trust on what the MoH says about malaria vaccine (χ2=7.160, df=1, P.=0.007), source of verification of negative information on malaria vaccine (χ2=15.368, df=3, P.=0.002), knowledge of any group or leaders, or individuals opposed to malaria vaccination (χ2=9.291, df=1, P.=0.002), awareness of people in my community opposed to malaria vaccine due to religion (χ2=8.224, df=1, P.=0.004) trust on malaria vaccine manufacturers to have good intentions for the child and other children in community(χ2=7.168, df=1, P.=0.007), having enough information about malaria vaccine and its safety (χ2=6.344, df=1, P.=0.012), confidence level in the safety of malaria vaccine (χ2=21.119, df=3, P.=0.000), trust in the country to manage risks associated with malaria vaccine side effects (χ2=4.441, df=1, P.=0.035) and trust in the health system to deliver malaria vaccine to your community (χ2=0.185, df=1, P.=0.667). At logistic regression analysis at multivariate level, 4 out of the 13 remained significant; Age of the child (AOR 0.634, 95% CI 0.418-0.962), information about malaria vaccine openly shared (AOR 4.085, 95% CI 1.671-9.987), source of verification of negative information about the malaria vaccine (AOR 1.573, 95% CI 1.120-2.207) and opposition to malaria vaccine linked to religion (AOR 0.581, 95% CI 0.352-0.958). The logistic regression model was statistically significant, χ2 = 37.076, p < .000. The model explained 11.6% (Nagelkerke R2) of the variance in vaccine uptake and correctly classified 65.9% of cases. Conclusions: Uptake of the first and second doses met WHO’s target coverage for vaccines, but uptake of the third and fourth doses do not. Malaria vaccine hesitancy is high, influenced by religion, confidence on the vaccine, open sharing of information and source of verification.
Keywords: Malaria Vaccine, Vaccine Hesitancy, Behavioural and Social Determinants
Cite this paper: Ezekiel Chepkiyeng, Alice Lakati, Anselimo Makokha, Predictors of Malaria Vaccine Hesitancy Among Caregivers in Bumula Subcounty, Bungoma County, Public Health Research, Vol. 13 No. 1, 2023, pp. 15-31. doi: 10.5923/j.phr.20231301.02.
1. Introduction
- BackgroundIn 2020, there were about 241 million malaria cases and 627 000 malaria deaths globally. Malaria case incidence increased from 59 per 1000 population in 2015 to 56 per 1000 population in 2019, before rising to 81 per 1000 population in 2020. The rise was linked to COVID-19 pandemic which disrupted malaria control services. A decade ago, there were 251 million cases, hence there was progress in reduction except for the Covid-19 pandemic 24) [24].Malaria is an important public health preventable disease in Kenya responsible for 13%-15% of outpatient cases. Its transmission and infection risks are influenced by altitude, rainfall patterns, and temperature, leading to huge difference in malaria prevalence seasonally and geographically. Approximately 70% of the Kenyan population is exposed to malaria, with 13 million and 19 million people in endemic and highland epidemic prone transmission areas, respectively. Malaria is a top cause of infant morbidity and mortality. Both children and pregnant women are at risk [11].WHO-recommended core package of several tools is in use for malaria prevention, diagnosis, and treatment. These tools include protection from bites using Insecticide-Treated Mosquito Nets (ITNs), ridding the households of mosquitos by Indoor Residual Spraying with insecticides (IRS), preventing malaria in pregnancy using intermittent preventive treatment (IPTp) and timely diagnosis and treatment of confirmed cases using Artemisinin-based Combination Therapy (ACTs).Recently, vaccination was identified as an additional tool to prevent malaria in children, making malaria a vaccine preventable disease (VPD). The Mosquirix (RTS, S) vaccine, which took 30 years to develop, has been clinically tried and is now in phased rollout [20]. Phase 3 clinical trials were done in 2009-2014 in a network of African research sites. Three of these sites are in Kenya and include Kombewa, Siaya and Kilifi. The trials involved more than 4,000 Kenyan children [15]. Children receiving four doses of RTS, S had reduced malaria and malaria-related complications compared to the control group [25]. Findings from the trial showed significant reduction of malaria in children, by 29%, including severe malaria and hospitalization [22].Malaria vaccine is now in phased rollout in 3 countries in Africa (Ghana, Kenya, and Malawi). In Kenya, the rollout is in selected sites in 8 counties which had malaria burden of more than 20% (Siaya, Busia, Bungoma, Kakamega Homa Bay, Kisumu, Migori and Vihiga) in 2019. RTS, S which is given at 6,7,9 and 24 months was officially launched in September 2019 for routine use in Kenya [15]. Statement of the Problem Malaria is a disease that threatens health and economy of the world. It is number nine among diseases in causing death and disability worldwide [2]. In 2020, there were about 241 million malaria cases and 627 000 malaria deaths globally. In Kenya, 70% of the population is exposed to malaria. WHO-recommended core package of several tools is in use for malaria prevention, diagnosis, and treatment. These tools include protection from bites using Insecticide-Treated Mosquito Nets (ITNs), ridding households of mosquitos by Indoor Residual Spraying with insecticides (IRS), preventing malaria in pregnancy using intermittent preventive treatment (IPTp) and timely diagnosis and treatment of confirmed cases using Artemisinin-based Combination Therapy (ACTs) [2].In 2019, a new tool was introduced to complement the existing package towards malaria prevention in children. This was the RTS, S malaria vaccine that has shown promising results from the clinical trials, pilot, and ongoing phased rollout. Vaccine Hesitancy (VH) and acceptability studies indicate inhibitors and promoters of new vaccine uptake that may hinder the roll out of the new malaria vaccine. A study done in Ethiopia on willingness by caregivers to accept malaria vaccine for their children showed that the level of acceptance was 32.3%, where marital status (AOR = 1.243), knowledge (AOR = 3.120), and earlier encounter of childhood vaccination (AOR = 2.673) were significant predictors of acceptance of malaria vaccine [3].A study done in Ghana, which is one of the three pilot countries, showed that full uptake was at 78%, which is below the WHO-recommended level of 90% [9].Studies done by Achieng et al. in Kenya on acceptability of the vaccine before rolling out revealed that an average 88% of the population approved the malaria vaccine for their own child and community. The highest acceptance would be 98.9% in malaria-endemic region, and lowest at 23% in the seasonal transmission areas [2].A study on perceptions of malaria vaccines before it was introduced in Kenya showed that uptake of malaria vaccine would be affected by culture, delivery of immunization services, level of education, existence of traditional methods to prevent malaria, gender, age, cohorts of caregivers and access. Most (94%) of the population with some schooling would accept the vaccine for a child, compared to 56% acceptance among those without schooling [22].Vaccine hesitancy (VH) is challenge in global health that the new vaccines face. Covid-19 VH was reported across the world, even among health care workers [18]. Polio vaccination was rejected in Northern Nigeria because of erroneous judgment by the religious leaders. Communities in Ghana rejected mass deworming due to misinterpretation of its intentions. Malaria VH has been reported in Ethiopia, Rwanda, and Uganda [9].The study therefore sought to establish the level of hesitancy for this malaria vaccine, and the associated predictors in Bumula Subcounty, Bungoma County.Research Questions1. What is the uptake level of the 4 doses of malaria vaccine?2. What is the prevalence of malaria vaccine hesitancy?3. What are the behavioural and social predictors of malaria vaccine uptake and hesitancy?General ObjectiveTo determine the predictors of malaria vaccine hesitancy among caregivers in Bumula subcounty, Bungoma County, Kenya.Specific Objectives 1. To determine the level of uptake of the 4 doses of malaria vaccine.2. To determine the prevalence of malaria vaccine hesitancy.3. To determine the behavioural and social predictors of malaria vaccine uptake and hesitancy.Significance of the StudyAvailable WHO-recommended control strategies against malaria are being faced with challenges due to drug and insecticide resistance and parasite undetectability, hence new and complementary tools such as malaria vaccine are timely to further reduce the disease burden. Kenya launched and introduced the malaria vaccine in phases in September 2019, to be an additional tool to control malaria, particularly among children. The study provided information on uptake and hesitancy levels of the malaria vaccine, with associated sociodemographic, interpersonal, contextual, and organizational predictors.This information obtained from of this study will be useful in developing strategies towards improving uptake to WHO-recommended levels in the pilot sites as well as when the vaccine will be rolled out across the country. The study will be useful to Bungoma County and Bumula subcounty which is in the malaria lake-endemic region, as they will utilize the results of this study to improve uptake.
2. Methodology
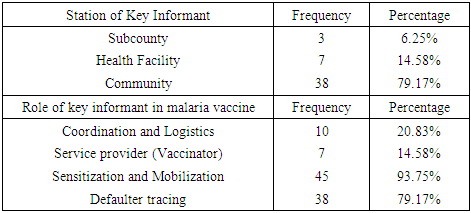
- Study designThis study utilized a cross-sectional design. Caregivers whose children were eligible for the four doses of the RTS, S vaccine as of December 2022 were recruited as participants. The participants were recruited, and data collection was done from 26th December 2022 to 20th January 2023. Prevalence of malaria vaccine hesitancy and associated predictors at the time of the study was determined. 38 CHVs and 10 KIs were also interviewed for qualitative data.Study siteBumula sub county is among the 10 sub counties in Bungoma County, which is in the malaria lake-endemic zone of Kenya. The subcounty is 345.2 km square, with a population density of 625 per km square. It has a population of about 220,000, with a total of 7 wards (KNBS, 2019). Males are about 105,000 while females are about 115,000. On average, each household has 4.8 members, which is higher than the national mean family size of 3.6 children. The population which lives below poverty line is 60% in the subcounty, compared to the national average of 53% (KNSS, 2021). The main economic activity is farming. The Bukusu tribe, which is a subset of the Luhya tribe, is the dominant group. Most of the population are Christians. Household heads are predominantly males, while females care for their children and conduct household chores. The mean age of marriage is 16 years for women and 18-27 for men. Bumula subcounty has 23 health facilities, with 18 public and 5 private facilities. The public facilities are one sub county hospital, five health centers, 12 dispensaries and 276 Community Units (CUs). The CHVs, who work in the 276 CUs are supervised by Community Health Extension workers. (CHEWs) and linked to the health facilities. The CHVs are trained to provide promotive, preventive, and basic treatment health services to children and the rest of the population, such as diarrhea, malaria, and pneumonia.Study populationThe caregivers of children who were eligible for all the four doses of the malaria vaccine between September 2019 and September 2022 were included in the study. The children whose caregivers were interviewed on their behalf, were 24 months to 46 months old by December 2022 since the fourth and final malaria vaccine dose is given at 24 months. Those younger than 24 months were too young to qualify for the fourth dose, while those older than 46 months were too old to have qualified for the first dose of malaria vaccine at 6 months when it was introduced in September 2019. The caregivers/children were identified from their attendance of the child welfare clinics (CWCs) clinics of the selected facilities. Qualitative data were collected through 4 FGDs and KIIs. The FGDs were conducted with 38 CHVs from the 19 CHUs linked to 4 of the 7 sampled health facilities. KIIs were conducted with 10 KIs from SCHMT and the 7 health facilities. Subcounty Community health strategy focal person (SCCHSFP), subcounty malaria control coordinator (SCMCC) and subcounty public health nurse (SCPHN) from the subcounty health management team (SCHMT) were interviewed, and 7 health care staff each from the 7 sampled facilities were interviewed.Inclusion criteriaCaregivers of children eligible for the four malaria vaccine doses between September 2019 and December 2022 attending the health facilities sampled were included. Exclusion criteriaCaregivers of children who took part of the vaccines in another facility were excluded as it was hard to verify the uptake of the doses in the CWC register. Caregivers of children who were extremely sick during the interview day at the facility were also excluded to enable them to attend to the child. Sample SizeCochrane’s formula was used to determine the sample size of children whose caregivers were recruited into the study.
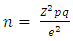 | (1) |
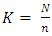 | (2) |
3. Results
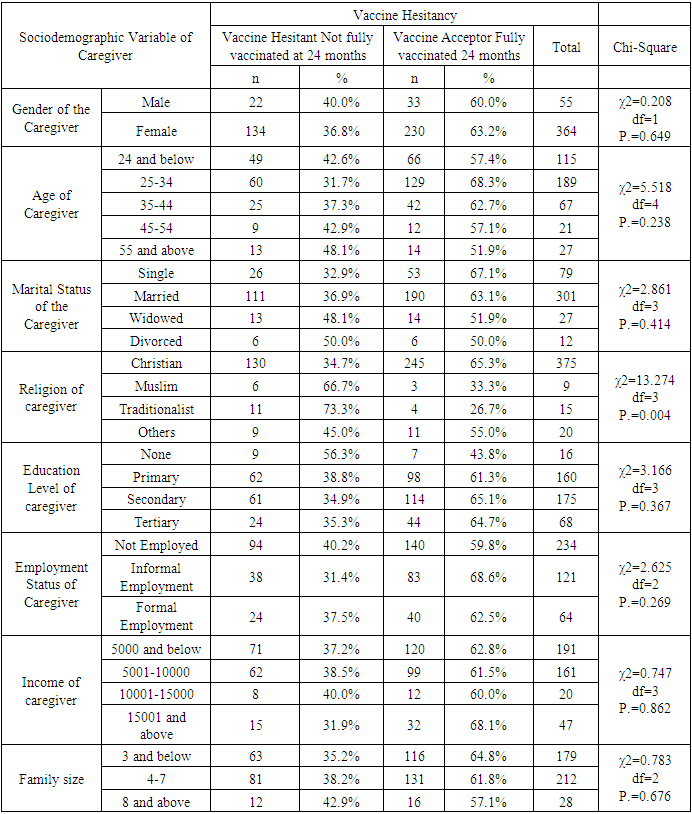
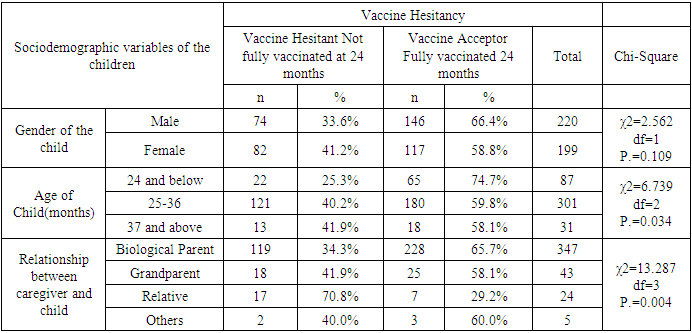
- Characteristics of RespondentsA total of 419 caregivers and their children, ten key informants and 38 FGD participants were recruited into the study.Sociodemographic Characteristics of the CaregiversA total of 419 caregivers and 38 key informants participated in the study. Majority (86.9%) of caregivers were female while 13.1% were male. The mean age of caregivers was 31.31 years (24, 35 years), and ranged from 17 to 80 years. 45.1% of the caregivers were 25-34 years, while 27.4% were below 24 years, and 27.4 were above 35 years. The majority (71.8%) of the caregivers were married, while 18.9% were single, 6.4% widowed and 2.9% divorced. Majority (89.5%) of the caregivers were Christian. 55.8% of the caregivers were not employed, while 28.9% had informal employment and 15.3% formally employed. 38.2% of the caregivers had primary education as their highest level of education, while 41% had secondary education, 3.8% had no formal education and 16.2% had tertiary education.The mean number of members per family was 4.24, with a range of 2 to 14 members. Half (50%) of the families had 4-7 members, while 42.7% had below 3 members. Average monthly income of the caregiver is 9179.71(3000, 6000 Kenya shillings), with a range from 0 to 300,000 Kenya shillings. 50% of the caregivers had a salary of 5,000 and below, while 38.4% had salary of between 5001-10000. Details of the distribution of the socio-demographic characteristics of caregivers are shown in Table 1.Sociodemographic characteristics of the childrenAbout half (52.5%) of the children were male while 47.5% were female. The mean age of the children was 29.32 months and ranged from 24 and 46 months. Most (71.8%) of the children were between 25-36 months, while 20.8% were below 2 years, and the rest (7.4%) were above 3 years.Most (82.8%) of caregivers were biological parents, while 10.3% were grandparents and 5.7% relatives.Details of the distribution of the socio-demographic characteristics of caregivers are shown in Table 2 below.Malaria Vaccine Uptake Full uptake of the malaria vaccine was 62.8%, while partial uptake was at 34.8% (1 dose at 1.4%, 2 doses at 9.5% and 3 doses at 23.9%) and no uptake at 2.4%. Uptake of the first dose was 97.6%, which reduced to 96.2% for second dose, 86.6% for the third dose and finally 62.8% for the fourth dose. The uptake is as shown in Figure 1 below.
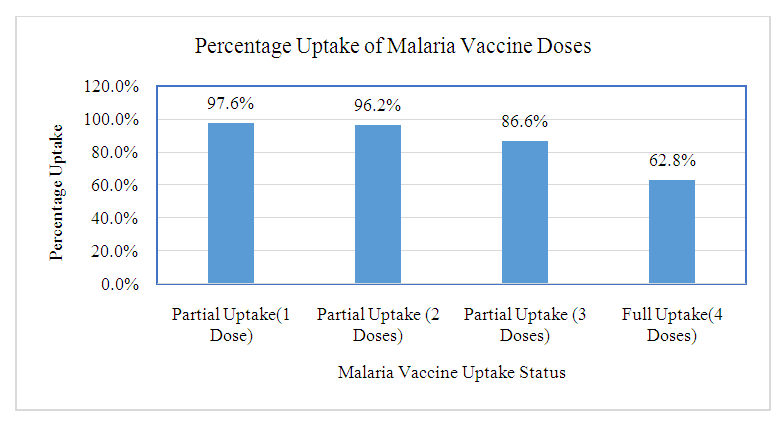 | Figure 1. Malaria Vaccine Uptake |
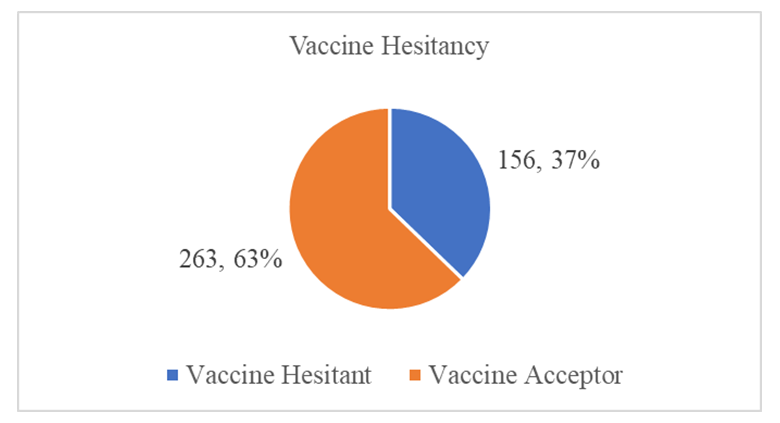 | Figure 2. Malaria Vaccine Hesitancy Chart |
|
|
|
|
|
|
|
|
4. Discussion
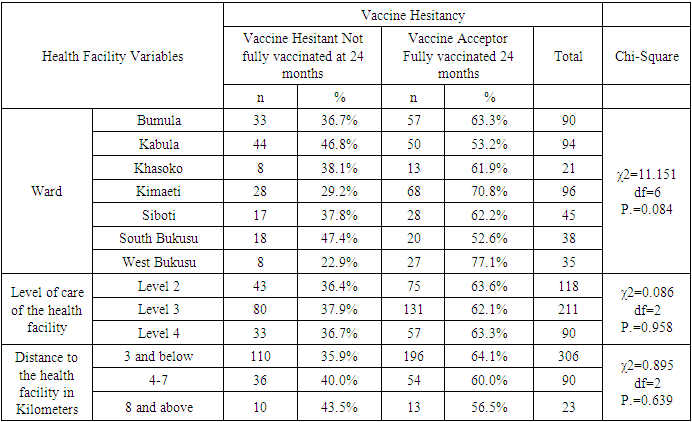
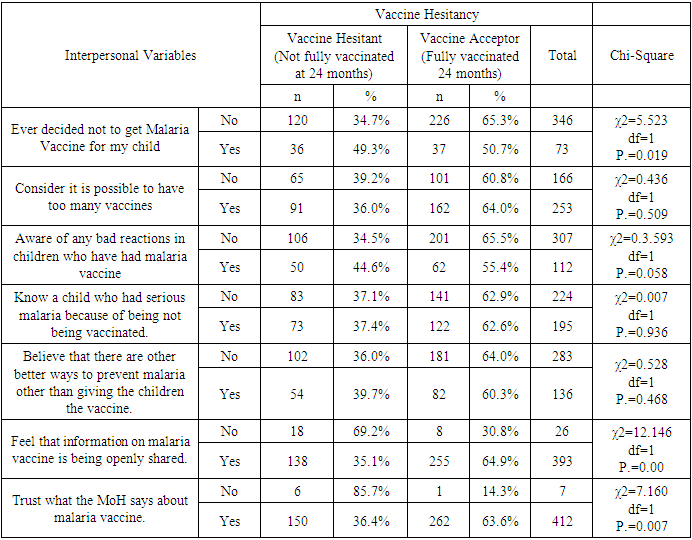
- Uptake of the first and second dose met the WHO target of 90%, while uptake of third and fourth dose did not. Reduced uptake of subsequent doses of the vaccine is similar to that observed in Ghana, where uptake of the three doses was 94.1% for RTS,S 1; 90.6% for RTS,S 2; and 78.1% for RTS,S 3 [22].This reduction in uptake of subsequent doses of the vaccine has also been observed in other vaccines in in Ghana [1], Cameroun [8], Senegal [13], Togo [1], Nigeria [7] and Congo [5]. A study done in Togo in 2016 showed complete immunization coverage was 72.3% (95% CI 69.7–74.8), which is higher than 68.2% in this study [1], but still below WHO recommendation. A cross-sectional study in Nigeria in 2016 revealed 58 % of children were fully immunized, which is lower than 68.2% found in this study, and still below WHO and national level targets [7].The uptake from this study of 62.8% is lower than what Achieng et al. found in Kenya in a study on acceptability of the vaccine before rolling out predicted, where an average of 88% of the population approved the malaria vaccine for their own child and community, ranging from 98.9% in malaria-endemic region, to 23% in the seasonal transmission areas [2]. Another study done in Ethiopia on willingness to accept malaria vaccine among caregivers of under-5 children in Southwest Ethiopian predicted a low uptake of 32.3% among caregivers, which is lower than the 62.8% in this study [3].This study identified some of the challenges with uptake of malaria vaccine similar to the challenges faced, and lessons learned, during the planning and early implementation of the RTS,S/AS01E vaccine in three out of the six regions that implemented the programme in Ghana [9]. A study in Cameroon on vaccine coverage and determinants of incomplete vaccination in children aged 12-23 months in, Cameroon revealed that longer distance from the vaccination centers was marginally significant [8], and another study in Togo found that children whose parents had to walk half an hour to one hour to reach a healthcare center were 57% (aOR = 1.57, 95% CI 1.15–2.13) more likely to have an incomplete immunization coverage than those whose parents had to walk less than half an hour [5]. This study contradicted these two studies, as distance from the health facility of the caregiver was not significantly associated with vaccine hesitancy (χ2=0.895, df=2, P.=0.639) [8]. Despite the insignificance of the association, the longer the distance from the health facility, the higher the vaccine hesitancy, with 35.9% of the caregivers 3km and below being vaccine hesitant, compared to 40% of those 4-7km away, and 43.5% of those 8 km and above away from the health facility. Despite decrease in vaccine hesitancy with increase in education, education of caregiver was not significantly associated with vaccine hesitancy (χ2=3.166, df=3, P.=0.367). This result contradicts findings from a study in Congo were children of mothers with secondary or higher education (AOR: 1.32; 95%CI: 1.00, 1.81) had significantly higher odds of being fully immunized compared to their counterparts whose mothers were less educated [19]. Another study on determinants of complete immunization among Senegalese children aged 12-23 months found that caregivers who attended at least secondary education level was a predictor of full childhood immunization (AOR 1.8 95% CI 1.20-2.48) [13]. Additionally, studies by Tabiri et al., and Ngeno et al and Acharya et al. found out that a higher educated parent was associated with higher odds of complete uptake both in the univariate analysis and the multivariate analysis (AOR: 4.72, 95% CI 1.27–17.55) [16]. Feeling that information about malaria vaccine was shared openly was significantly associated with vaccine hesitancy (AOR 4.085, 95% CI 1.671-9.987), which is similar to findings from a study in Nigeria on immunization coverage and its determinants among children, were access to immunization information (aOR = 1.8, 95% CI = 1.1-2.5) and mothers having good knowledge of immunization (aOR = 2.4, 95% CI = 1.6-3.8) were significant determinants of full immunization [7].Income was not significant predictor of vaccine hesitancy in this study (χ2=0.747, df=3, P.=0.862), which contradicts findings from a study done on incomplete immunization among children aged 12-23 months in Togo, were the likelihood of incomplete immunization in children decreased with the increase in household's income (aOR = 0.73, 95% CI 0.58-0.93) [5]. Another study on individual and community level determinants of child immunization in the Democratic Republic of Congo found out that children from the richest wealth quintile had significantly higher odds of being fully immunized compared to their counterparts whose mothers were relatively poorer (AOR: 1.96; 95% CI: 1.18-3.27) [19].Whereas marital status, was not significantly associated with vaccine hesitancy in this study, findings from a study done by Asmare et al in Ethiopia showed that marital status (AOR = 1.243; 95% CI 1.021–3.897) was significantly associated with willingness to accept a malaria vaccine for their children [3]. Additionally, the study found knowledge (AOR = 3.120; 95% CI 1.689–5.027), and previous experience with childhood vaccination (AOR = 2.673; 95% CI 1.759–4.101) significantly associated with willingness to accept a malaria vaccine for their children, while this study found knowledge((χ2=3.166, df=3, P.=0.367) and previous experience with vaccines ((χ2=1.647, df=1, P.=0.199) not significant.
5. Conclusions
- Uptake of the individual doses varies, with first and second dose meeting the WHO target of 90%, while uptake of third and fourth dose did not. Full uptake is below recommended target, and vaccine hesitancy is therefore high. Despite the uptake having a slow start and high hesitancy at the beginning, there has been improvement of uptake and decrease in hesitancy over time, even though it is yet to meet WHO recommended levels. Despite promising results in reducing malaria burden, malaria vaccine uptake faces an uphill task with the behavioral and social drivers of uptake. Social and behavioral determinants of vaccine hesitancy include age of the child, information about malaria vaccine openly shared, source of verification of negative information about the malaria vaccine and opposition to malaria vaccine linked to religion.
6. Recommendations
- Due to the 3rd and 4th dose not meeting WHO target, action from all stakeholders is required to improve the uptake in preparation for scale-up to other sub counties and the rest of the country. The county and immunization program need to implement suggestions shared by the respondents. Health promotion need to be done, targeting and through religious institutions. Mobilization, outreach, and follow-up targeting the older children eligible for 3rd and 4th vaccine need to be strengthened. There is need for a longer, more intensive, and sustained delivery of contextually appropriate sensitization prior to implementation of a programme such as MVIP. More research needs to be undertaken within same location and other areas where the vaccine is being implemented to get more insights on the predictors of vaccine hesitancy. There is a lot of work needed to improve uptake of vaccines to WHO-recommended levels. With Covid-19 uptake having faced similar challenges even among health care workers, the socio-demographic predictors of vaccine uptake need to be studied more to enable interventions that address them. Due to the time and scope limitations of this study, it is recommended that further research over a long period and with a bigger sample be done to ascertain the findings.A channel for verifying negative information needs to be established, such as a toll-free line. Promotional activities should be done through the religious institutions and leaders. More open sharing of information about malaria vaccine should be done through all channels of communication.More scientific research needs to be done to ascertain whether the fourth vaccine can be either removed or moved to a lower age.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML