-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Public Health Research
p-ISSN: 2167-7263 e-ISSN: 2167-7247
2021; 11(5): 141-146
doi:10.5923/j.phr.20211105.02
Received: Nov. 15, 2021; Accepted: Dec. 1, 2021; Published: Dec. 15, 2021

Sociodemographic Gradients in Periodontal Health Among Geriatric Kenyans
Walter Ogutu Amulla1, Aaron Gichaba Misati2, Euginia Makinia Wekesa3
1Department of Public Health, National Yang Ming Chiao Tung University, Taipei, Tawan
2Department of Public Health, Kabarak University, Kenya
3Department of Microbiology, University of Nairobi, Kenya
Correspondence to: Walter Ogutu Amulla, Department of Public Health, National Yang Ming Chiao Tung University, Taipei, Tawan.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Periodontal disease remains a highly prevalent oral disorder globally, disproportionately affecting socially-disadvantaged members of society. However, most epidemiological studies focus on younger age-groups leaving the elderly underexplored. Objectives: The aim of this study was to explore sociodemographic gradients in periodontal health of elderly Kenyans. Methods: data from a cross-sectional study conducted among 300 respondents aged 65 plus years was analyzed. Respondents were selected by stratified sampling technique. Periodontal health was assessed using self-reported gingival bleeding and tooth mobility. Chi-square was used to determine whether distribution of periodontal outcomes differed significantly across categories of sociodemographic factors, with significance set at p<.05. Results: Gender distribution was 55.3% females against 44.7% males. Gingival bleeding was reported by 60% of respondents while 56% reported tooth mobility. The distribution of these periodontal outcomes varied significantly (p<0.05) with residence, educational attainment, occupational history, household composition and economic status. Strongest variations (p<0.001) in periodontal health were found with residence and occupational history. Conclusion: Significant sociodemographic gradients existed in periodontal health of elderly Kenyans. Our findings suggest that health disparities occur even within minute socioecological contexts. Focus on reducing social disparities in periodontal health among the elderly is recommended.
Keywords: Self Report, Aged, Gingival Hemorrhage, Socioeconomic Factors, Tooth Mobility, Kenya
Cite this paper: Walter Ogutu Amulla, Aaron Gichaba Misati, Euginia Makinia Wekesa, Sociodemographic Gradients in Periodontal Health Among Geriatric Kenyans, Public Health Research, Vol. 11 No. 5, 2021, pp. 141-146. doi: 10.5923/j.phr.20211105.02.
Article Outline
1. Introduction
- Periodontal disease remains a highly prevalent oral disorder globally with risks increasing notably as individuals become old [1]. The disease manifests in array of symptoms extending from bleeding gums and calculus to gingival recession and mobile teeth. When left untreated, periodontal disease ultimately leads to loss of teeth, affecting both physiological, psychological and aesthetic quality of life [2,3].Reported prevalence of periodontal disease vary widely across countries, ranging from 36-67% among adult populations in low-and-middle-income countries [4]. Specific studies among elderly have reported prevalence of 61% in Georgia [5], 45% in Korea [6], 53-70% in China [1], 62% in the United States [7], 46% in South Africa [8] and 79% in Nigeria [9].Data on geriatric periodontal disease prevalence remains sparse in Kenya. However, extant studies paint a grim picture of the status of periodontal health in the country. One such study reported a 90% prevalence among adults [10], while another reported prevalence ranging between 73-94% using the periodontal screening and recording (PSR) technique [11]. Some of the dental problems among the elderly reported in a study in Nairobi included dental plaque (89.9%), calculus(85.6%), gingival recession (82.5%) and bleeding gums (77.4%) [12].Epidemiological studies have associated periodontal disease with a myriad systemic health conditions including cardiovascular disease, diabetes, Alzheimer’s and rheumatoid arthritis [13–15]. Other studies established association between periodontal disease and certain forms of cancers as well as respiratory tract infections [16,17].Evidence is emerging that social gradients exist in prevalence of periodontal disease across populations, disproportionately affecting socially-disadvantaged members of society [18,19]. However, most studies focus on younger age-groups leaving the elderly underexplored [20,21]. Extant geriatric studies in this respect are meager and inconsistent and are done mainly in high income countries [22,23]. In practice most researchers control for sociodemographic characteristics instead of examining them [24]. As such little is extant on such gradients among the elderly in Kenya. The aim of this paper was to determine whether significant sociodemographic gradients occurred in periodontal status of elderly Kenyans.
2. Materials and Methods
2.1. Study Design and Setting
- This was a cross-sectional study done in a rural community in Homa Bay county, South-western Kenya. The community is located about 30km north-east of Homa Bay town.
2.2. Study Participants
- Three hundred (300) respondents were involved in this study randomly sampled from a list of 1159 elderly (aged ≥65) dwelling in the study area. This is the cut-off age for geriatric population in Kenya. The population is of interest due to expansion of morbidity in Kenya and lack of comprehensive geriatric healthcare policy. The sample size was determined using Yamane (1967) formula. Respondents were drawn from four administrative locations using proportionate stratified sampling technique.
2.3. Measures
- A structured questionnaire was administered to collect data on sociodemographic characteristics and periodontal status. Sociodemographic data included age, gender, geo-administrative residence, education, occupational status, household composition and self-rated economic status. Periodontal status was determined by two questionnaire items assessing [i] self-reported gingival bleeding and [ii] tooth mobility, in a dichotomous (yes/no) response format. The questionnaire was pre-tested with 30 elderly mirroring the study population prior to the main study.
2.4. Data Analysis
- Statistical analyses were performed on SPSS version 25. A composite binary variable was computed to collate cases with both gingival bleeding and tooth mobility. Descriptive data was summarized by frequencies and percentages. Chi-square test was used to determine whether distribution of periodontal outcomes differed significantly across categories of sociodemographic factors at p-value < 0.05 (two-tailed).
2.5. Ethics Statement
- Ethical clearance was granted by the Institutional Review Board (IRB) of the University of Eastern Africa, Baraton (IRB no. UEAB/REC/21/10/2019). Informed consent was confirmed by the IRB and obtained from participants before administering questionnaires. Data identifying respondents’ geo-administrative residences were anonymized in the presentation of findings.
3. Results
3.1. Sociodemographic Characteristics
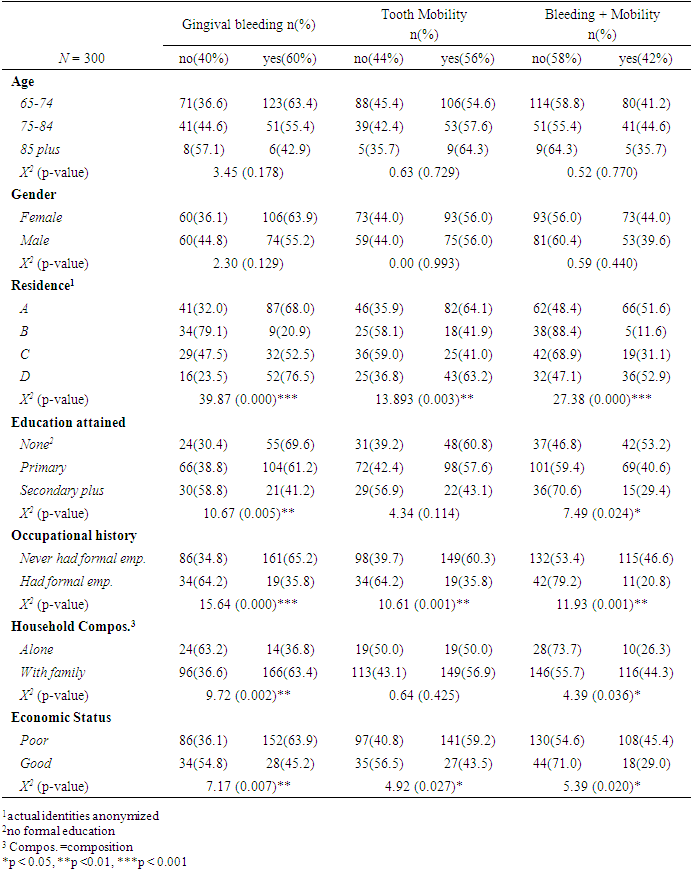
- As collated in [Table 1], over 6 in 10 respondents were in the age bracket of 65-74 years, with females accounting for more than half (55.3%) of the study sample. Only 17% of the respondents had secondary education and above with nearly the same proportion (17.7%) reporting not having had formal employment. A small proportion (12.7%) lived alone and nearly 8 in ten (79.3%) reported poor economic status. Respondents were disproportionately distributed across geo-administrative residences with residence B accounting for the least (14.3%).
|
3.2. Distribution of Periodontal Outcomes by Sociodemographic Factors
- A summary of prevalence and distribution of periodontal across sociodemographic variables is delineated in [Table 2]. Gingival bleeding was the leading periodontal outcome with 3 in 5 (60%) respondents reporting cases. Still, more than half (56%) reported having at least one mobile tooth, while 2 in 5 (42%) reported both bleeding gums and tooth mobility.
|
4. Discussion
- Social determinants of health (SODH) remain a leading source of health disparities across populations [25]. Northwood and colleagues (26) asserted that up to three-quarters (75%) of health disparities are a result of SODH. Although some studies have attempted to explore social disparities in periodontal status [27,28], little is extant on elderly Kenyans.
4.1. Prevalence of Periodontal Outcomes
- We investigated the distribution of geriatric periodontal health outcomes across categories of sociodemographic factors to determine whether significant variations occurred. The most prevalent periodontal outcome was gingival bleeding reported by 60% of the elderly involved in the study. As the leading sign of periodontitis worldwide, gingival bleeding is an important indicator of the status of periodontal health and often occurs alongside tooth mobility and gingival recession [20]. Self-report of these outcomes have been used to determine burden of periodontal disease among community-dwelling individuals [29].This finding thus underscores a glaring burden of periodontal disease among the studied population. Indeed more than half (56%) of the study respondents indicated suffering from tooth mobility and a further 42% reported comorbidity of bleeding and mobility. Reported prevalence of periodontal disease vary widely across countries, ranging between 36-67% among adult populations in low-and-middle-income countries [4]. The findings of this study therefore indicate appreciably high prevalence in comparison.
4.2. Sociodemographic Gradients in Periodontal Health
- In chi-square analyses, we discovered that respondents’ area of residence, educational attainment, occupational history, household composition and economic status significantly related with periodontal outcomes in the studied population. By and large, the distribution of these periodontal outcomes were such that higher prevalence occurred among poorer sociodemographic measurement levels. There was significantly higher prevalence of bleeding gums (69.6%) among respondents with no formal education compared to those with primary (61.2%) and secondary (41.2%) education (p=0.005). This pattern was replicated for comorbidity with tooth mobility as well (p=0.024). A similar pattern was observed with occupational history, whereby significantly higher prevalence of bleeding gums, mobility and comorbidity occurred among respondents who never had formal employment (65.2%, 60.3%, 46.6%, respectively) compared to those that were formally employed (35.8%, 35.8%, 20.8% respectively). This was true also for self-rated economic status, with respondents in the “poor” category having higher prevalence of bleeding gums (63.9% vs. 45.2%), tooth mobility (59.2% vs. 43.5%) as well as comorbidity (45.4% vs. 29.0%). This disaggregation of periodontal outcomes by sociodemographic attributes revealed disproportionate burden of dental morbidity even among the elderly in rural communities. This indicate that social determinants of health significantly contribute to disparities in periodontal status of rural elderly in Kenya. The strength of this finding is the revelation that even within minute socioecological contexts, health disparities occur across sociodemographic gradients. The disproportionate distribution of health across social gradients is a common phenomenon the world over and has been documented for a number of diseases and causes of mortality. Extant geriatric studies have reported evidence of social gradients in the oral health of the elderly population, albeit in different socioeconomic contexts [22,23]. Our findings confirm that social gradients in periodontal health exist among elderly communities in in rural Kenya. It is reported that social disparities affect oral health by acting in a complex web of causation involving oral hygiene behavior, knowledge, attitudes and dental care access, and that adjusting for these mediatory attributes statistically diminishes the social gradient [21].Education-health gradient is explained by the influence education has on cognitive empowerment and knowledge acquisition which increases an individual’s sense of control and improves health seeking behavior. Moreover, educational attainment, particularly in emerging economies, often also denotes better employment opportunities and greater incomes thus reducing gradients associated with economic status [21]. Our findings underscore, therefore, the need for sensitization and economic empowerment among elderly communities. Furthermore, since dental health outcomes among the elderly denote cumulative effects of lifetime exposure, efforts to address social disparities throughout the lifecycle could have greater benefits for affected communities.The disparities across categories of household composition could be explained by lower economic stress among lone-dwelling elderly or dental neglect leading to low self-perception of gingival bleeding and tooth mobility. Geospatial disparities reported in this study did not follow an exactly linear pattern but a trend was observed in which residents of locations A and D consistently reported higher prevalence of periodontal morbidity compared to the rest. This could be due to contextual and environmental factors not investigated and warrants further research.
4.3. No Age or Gender Disparities
- This study found no statistically significant difference in the distribution of periodontal outcomes between males and females, indicating there were no gender disparities in periodontal status. Earlier review evidence suggested the prevalence of periodontitis was lower in females compared to males (28.1% vs. 37.4%). However, the authors admitted that the overall effect of sex in the meta-analysis was comparatively small [30]. On the other hand, a meta-analysis of studies conducted among elderly Chinese did not find gender differences in prevalence of gingival bleeding [1]. Our findings mirrored this meta-analysis, with prevalence being similar or slightly and insignificantly lower in males. Additionally, age disparities in periodontal status were not statistically significant in this study. We posit that since these were elderly 65+ years old, the manifestation of periodontal disease as assessed in the study was not different, particularly considering the aggregation of the age-groups. This finding concurred with the findings of Fukuda and colleagues [31] who reported no significant difference in periodontal outcomes across gender among a Kenyan elderly population.
5. Conclusions
- This study investigated sociodemographic disparities in periodontal health among the elderly in rural Kenya. We discovered significant disparities across respondents’ area of residence, educational attainment, occupational history, household composition and self-rated economic status. No age or gender disparities in periodontal status were observed. Gradients in periodontal status across geo-administrative units raised questions on the role of environmental and structural factors in periodontal health in the studied population. We recommend adult education and sensitization, economic empowerment for the elderly and a life cycle approach to addressing social disparities in periodontal health.
6. Limitations
- Periodontal disease is variously assessed with studies citing lack of consensus. In this study periodontal status was assessed by self-report, which admits some bias in regards to illness recognition. Nonetheless, this is an established methodology in epidemiological studies and has been reported to closely mimic clinical assessments [20,32].Furthermore, this study focused on establishing social gradients rather than causality. The results should therefore be interpreted within the context of the analysis.
ACKNOWLEDGEMENTS
- The authors acknowledge the support of administrative chiefs who assisted with the community entry during data collection.
Declarations
- Funding: no external funding.Conflict of interest: The authors have no conflicts of interest to declare for this study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML