-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Public Health Research
p-ISSN: 2167-7263 e-ISSN: 2167-7247
2021; 11(1): 19-32
doi:10.5923/j.phr.20211101.03
Received: Jan. 27, 2021; Accepted: Mar. 8, 2021; Published: Mar. 20, 2021

Health-Care Spending Attributable to Cervical Cancer: A Systematic Review
Abdi Shale Abdi1, Wanja Mwaura-Tenambergen2, Job Mapesa3
1Health Information Specialist, Ministry of Health, Garissa, Kenya
2Department of Health Systems Management, School of Medicine and Health Sciences, Kenya Methodist University, Nairobi, Kenya
3Department of Public Health Human Nutrition and Dietetics, School of Medicine and Health Sciences, Kenya Methodist University, Nairobi, Kenya
Correspondence to: Abdi Shale Abdi, Health Information Specialist, Ministry of Health, Garissa, Kenya.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Cervical cancer is the fourth most common cancer globally, due to an extremely low rate of screening and prohibitive prevention costs. Knowing the costs of screening will help planners and policymakers design, implement, and scale programs. The objective of this review is to quantify health care spending attributable to cervical cancer in sub-Sahara Africa. We searched PubMed and Google Scholar for English language publications detailing cost analyses of Human Papilloma Virus vaccination, different cervical cancer screening methods and pre-cancer treatment globally. The main outcome of interest was the cervical cancer prevention cost per woman. Expenditure data were extracted and a descriptive review was conducted for each included study. Among the screening strategies, Visual Inspection with Acetic acid (VIA) was the least expensive. In addition, preventative Cryotherapy without screening was the least expensive strategy for preventing cervical cancer in HIV-infected women compared to other strategies and their combinations. The screening costs for Pap, LEEP and colposcopy were relatively high. HPV Vaccination cost the highest among the cervical cancer prevention strategies reviewed. The varying costs for these proposed strategies provide options for program implementers including donors, insurance firms, and the Ministry of Health to efficiently plan based on the anticipated screening treatment coverage and program budgets.
Keywords: Cervical Cancer, Direct Medical Expenditure, Cancer Prevention, Financial cost, Economic costs
Cite this paper: Abdi Shale Abdi, Wanja Mwaura-Tenambergen, Job Mapesa, Health-Care Spending Attributable to Cervical Cancer: A Systematic Review, Public Health Research, Vol. 11 No. 1, 2021, pp. 19-32. doi: 10.5923/j.phr.20211101.03.
1. Introduction
- The prevailing drive and enthusiasm to achieve Universal Health coverage present an incredible opportunity to save the lives of women globally from cervical cancer. Health financing is crucial for achieving universal health coverage by raising adequate funds for health and rendering financial risk protection [1]. Cost concerns play an important role in whether or not women are screened and treated for cervical cancer. Moreover, cost concerns also limit strategies to ensure effective treatment for screen-positive women, which is an essential part of the cervical cancer prevention cascade. Generating practical data on the cost of alternative models of service delivery is imperative, as countries grapple with tough decisions about which interventions can be effectively implemented using innovative financing mechanisms and promote sustainability in the face of donor decline. Accurate cost estimates for preventive treatment for women who screen positive are pivotal for economic evaluations, policy decisions, and planning future medical care expenditures [2].Globally, cervical cancer is the fourth most common cancer with an estimated 570,000 cases and 311,000 deaths forecast in 2018 [3]. More than 80% of cervical cancers occur in developing countries, where vaccination, screening, and treatment are limited [4]. The World Health Organization estimates that between 10 and 11 million cancers will be diagnosed each year in low- and middle-income countries by 2030 if no significant investments in cervical cancer prevention are made now [5]. The global age-standardized incidence rate is 14.1 per 100,000 woman years, compared to 40.1 per 100,000 woman years in Kenya [6]. The disparities in cervical cancer incidence reflect differences in investment in, access to and uptake of cervical cancer prevention programs.Cervical cancer can be prevented through HPV vaccination and screening programs designed to identify and treat precancerous lesions known as high-grade squamous intraepithelial lesions (SIL) [7] [8]. Various technologies have been developed to detect and treat precancerous lesions including Pap smear, colposcopy, visual inspection with acetic acid or Lugol’s iodine (VIA/VILI), HPV DNA testing, cone biopsy, Cryotherapy, and loop electrosurgical incision procedure (LEEP) [9] [10]. HPV vaccination programs are scaling up globally but implementation has been slow [11]. Besides, vaccination in most countries, mainly targets adolescent girls, leaving screening programs for women of reproductive age. High-risk human papilloma virus (HPV) testing [12] [13] [14] [15] and visual inspection with acetic acid (VIA) [16] [17] are recommended screening strategies that can be effectively coupled directly with preventive treatment in low-resource settings [18]. HPV testing has advantages over VIA, including a significantly higher sensitivity for precancerous lesions [19] [20] and a definitive result that allows for a simplified protocol with clear management options. However, there is no currently available point-of-care test for HPV, making same-day treatment strategies impossible [21]. Also, ensuring that women who test positive with VIA or HPV have access to safe, effective, and affordable treatment is crucial to reducing their risk for cervical cancer [22]. Cryotherapy is a simple means of treating women with precancerous cervical lesions [23] [24]. In low-resource settings, Cryotherapy is cost-effective and affordable, making it an ideal first-line treatment for visible lesions or cervical pre-cancer [25]. LEEP stands for Loop Electrosurgical Excision Procedure. It’s a treatment that prevents cervical cancer. A small electrical wire loop is used to remove abnormal cells from your cervix. LEEP surgery may be performed after abnormal cells are found during a Pap test, colposcopy, or biopsy [26].Financial issues can play an important role in whether or not women are screened and treated for cervical cancer. Women with lower incomes and those without health insurance are less likely to be screened [27]. This systematic review examined the cost of different cervical cancer prevention strategies globally which is beneficial in planning the scale-up of cervical cancer prevention and ultimately achieving Universal Health Coverage. It provides cost estimates required to finance cervical cancer prevention.
2. Methods
- The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed in conducting this systematic review. This systematic review was not registered with PROSPERO.Search strategy.A systematic search for published literature in English was conducted on PubMed. Grey literature search was also conducted through the search engine Google Scholar. References of retrieved articles and reports were screened to identify additional potential published and unpublished studies. The key words used in the search were cervical cancer, prevention, costing, cost analysis, and their synonyms. The following search strategy was used; (((((cost) OR (healthcare cost)) AND (uterine cervix cancer)) OR (uterine cervix carcinoma)) OR (uterine cervix adenocarcinoma)) AND (Prevention))))). The PubMed search yielded a total of 115 studies. Non-empirical studies (commentaries, editorials, etc.) and studies that did not explicitly assess cost of the implementation strategies (knowledge, attitudes, and beliefs; incidence and prevalence; safety and efficacy) were excluded from the systematic review See figure 1 below.
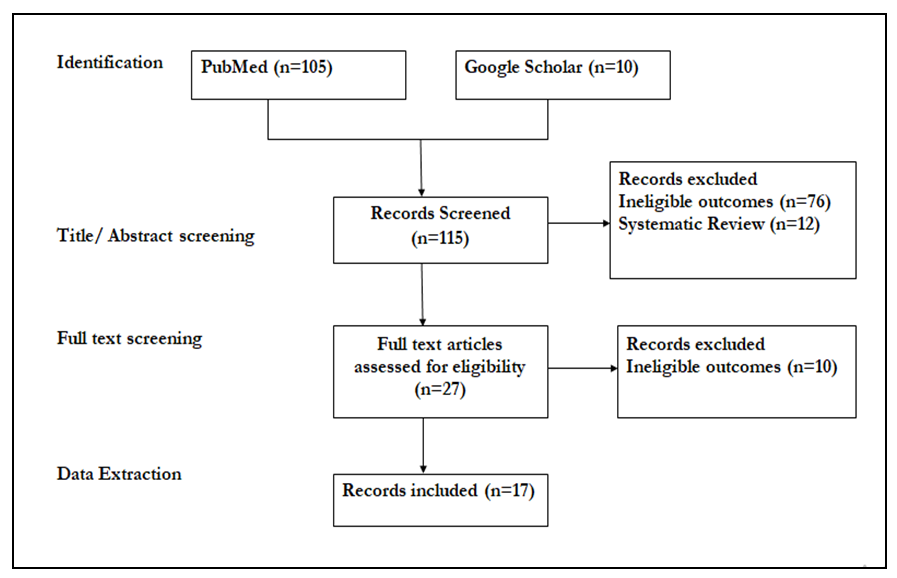 | Figure 1. Literature screening process |
3. Results
- Study characteristics are summarized in Table 1. The studies included were published between 2005 and 2019. The 17 studies were conducted in 30 sites, 25 in sub-Saharan Africa, two in Southern Asia, and three in South America. While majority of studies (16) included in the review only estimated economic costs, one estimated both financial and economic costs of cervical cancer prevention. Seven publications reviewed were based on secondary data while the ten studies were based on primary data. The publications reviewed estimated the costs of VIA, Papanicolaou smear (Pap), HPV vaccination, HPV DNA screening, and Colposcopy/Biopsy as cervical cancer screening strategies. The studies also estimated the costs of Cryotherapy or Loop electrosurgical excision procedure as treatment strategies for pre-cancer.
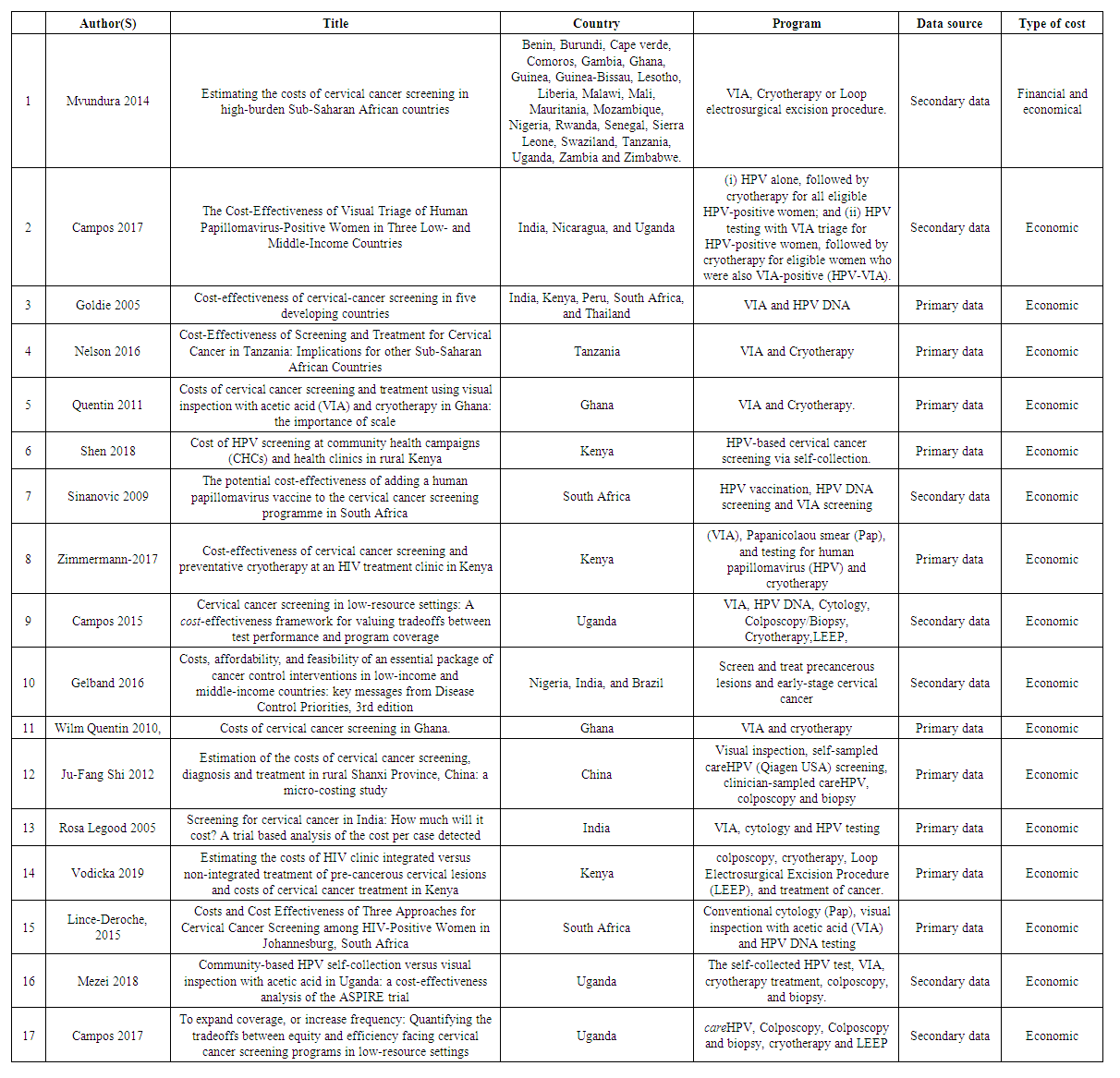 | Table 1. Study characteristics |
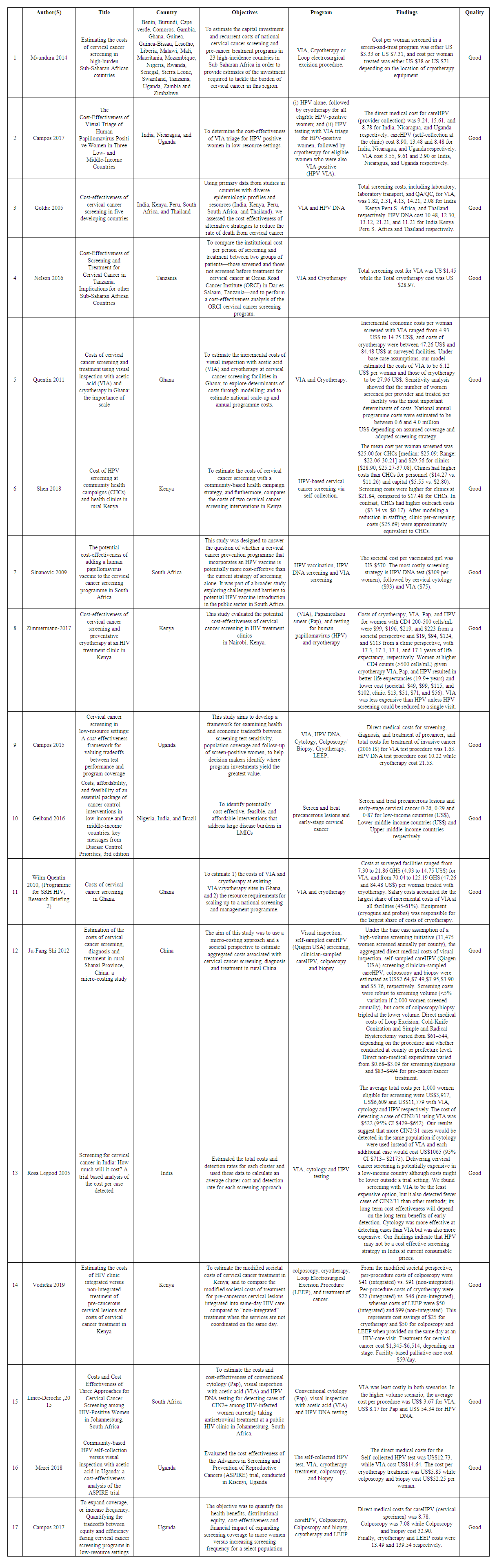 | Table 2. Table of evidence |
4. Discussion
- The findings of this review show that among the screening strategies, VIA was the least expensive. In addition, preventative Cryotherapy without screening was the least expensive strategy for preventing cervical cancer in HIV-infected women compared to VIA, Pap, HPV, and their combinations. The screening costs for Pap, LEEP and colposcopy were relatively high. HPV Vaccination cost the highest among the cervical cancer prevention strategies reviewed.The variability in costs can be attributed to a number of factors including: strategy, such s those for laboratory equipment and supplies, specimen transport, and training for and supervision of particular techniques. The variability can also be attributed variations in underlying assumptions, methods for cost calculations, and data availability for each study. The perspective adopted by a study influences the cost per woman screened as reported in Sinanovic et al (2009) and Zimmermann et al (2017). Studies that adopted the societal perspective reported higher costs compared to those that adopted the provider perspective.Variability in the cost per woman screened and treated may also be possible through economies of scale. By spreading fixed costs (capital goods and equipment) over increased screening participants, the screening and treatment costs reduce. In addition, cost reductions can be attributed to bulk purchases, sharing of services, and reduced personnel downtime. Recent studies have found that large-scale HIV prevention and treatment programs are associated with decreased unit costs when scaled up, across multiple countries [43] [44].Integration of cervical cancer prevention services into HIV treatment programs offers significant cost savings as reported by Vodicka et al (2019). This finding is consistent with that of a study in Zambia which found that increasing linkages and improving integration of HIV and sexual and reproductive health services led to more cost-effective service delivery [45]. However, it is important to come to an understanding as to whether integrated care is to be considered an intervention or whether it is to be interpreted, and evaluated, as a complex strategy to innovate and implement long-lasting change in the way services are delivered, including multiple changes at multiple levels.Single and two-visit scenario is a major cost driver. The costs of same day treatment of cervical precancer has also been shown to be less expensive by Zimmermann et al (2017) [35]. However, implementers should also be aware of potential diseconomies that may arise from longer wait times and disenrollment, and provider burnout due to expanding the services in a very resource-constrained setting [46].
5. Study Limitation
- This study does not estimate costs for linkage to treatment. Although this underestimates the total cost of cervical cancer prevention, our estimates are designed to be of direct budgetary and programmatic relevance to actual screening and treatment of precancer.
6. Conclusions
- To better plan effective cervical cancer prevention programs, a systematic review is needed to compare the cost-effectiveness of alternative screening and treatment programs. Moreover, new mathematical models have been developed to delineate the natural history of cervical cancer and assess the clinical benefits and cost-effectiveness of alternative cancer screening strategies. These models take into account differences in the relative effectiveness and requirements of various recruitment strategies, screening tests, treatment approaches, and follow-up protocols for a given country setting. Cost-effectiveness analysis of these interventions is crucial to understand how to stage these interventions for cervical cancer prevention.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML