-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Public Health Research
p-ISSN: 2167-7263 e-ISSN: 2167-7247
2020; 10(4): 119-122
doi:10.5923/j.phr.20201004.02
Received: Jul. 18, 2020; Accepted: Aug. 23, 2020; Published: Sep. 15, 2020

Breeding Sites of Mosquitoes (Diptera: Culicidae) at the University of Port Harcourt, Rivers State, Nigeria
Noutcha M. A. E., Nwokedike V. O., Asadu P. C., Okiwelu S. N.
Entomology and Pest Management Unit, Department of Animal and Environmental Biology, University of Port Harcourt, Port Harcourt, Rivers State, Nigeria
Correspondence to: Okiwelu S. N., Entomology and Pest Management Unit, Department of Animal and Environmental Biology, University of Port Harcourt, Port Harcourt, Rivers State, Nigeria.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

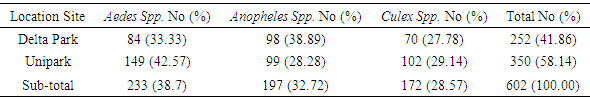
Malaria is the most widespread parasitic disease in the inter-tropics, especially in Sub-Saharan Africa. Lymphatic filariasis is a major cause of acute and chronic morbidity, affecting humans in tropical and sub-tropical countries. Both diseases involve culicid vectors. Integrated Vector Management (IVM) consists of use of insecticide treated nets (LLINTs), Indoor Residual Spray (IRS) and larviciding. A prerequisite to effective larviciding is an accurate knowledge of breeding sites. Breeding sites at two parks at the University of Port Harcourt were identified over a 2-month period; standard keys were used for immature and adult mosquito identifications. Mosquitoes utilized both containers and pool breeding site-types. Based on number of breeding site, the highest numbers were from tyres, puddle and plastic containers; lowest number per breeding site was from tin cans. However, these differences were not significant. More immatures were collected from University Park, the larger of the 2 parks, but the difference was not significant. In University Park, Aedes was the most abundant while Anopheles was least. In Delta Park, Anopheles was highest and Culex least. The differences were not significant. The varying choices of breeding sites across localities by species in the 3 genera (Aedes, Anopheles, Culex) highlighted the need for detailed investigations on preferred breeding sites at each location, prior to larviciding.
Keywords: Aedes, Anopheles, Culex, Breeding sites, University, Rainforest, Nigeria
Cite this paper: Noutcha M. A. E., Nwokedike V. O., Asadu P. C., Okiwelu S. N., Breeding Sites of Mosquitoes (Diptera: Culicidae) at the University of Port Harcourt, Rivers State, Nigeria, Public Health Research, Vol. 10 No. 4, 2020, pp. 119-122. doi: 10.5923/j.phr.20201004.02.
1. Introduction
- Malaria is the most widespread parasitic disease in the inter-tropics, especially in Sub-Saharan Africa [1]. According to the WHO, malaria affected 228 million worldwide in 2019, compared to 219 million in 2017, with 405,000 human losses [2]. Among the deaths attributable to malaria, 67% were children under the age of 5years [2]. In addition, Africa remains the continent most affected by the disease with 93% of malaria episodes and 74% of deaths [2]. While has been reduction in the malaria burden in a few countries, challenges of pyrethroid resistance and the need to target outdoor transmission make integrated vector management an appealing approach [3]. It is estimated that globally 1.2 billion people are at risk to lymphatic filariasis (Lf), a major cause of acute and chronic morbidity, affecting humans in tropical and subtropical countries [4]. There are 3530 species of mosquitoes in 43 genera of the family Culicidae. They are divided into 3 subfamilies: Anophelinae (Anophelines), Culicinae (Culicines) and Toxorhynchitinae. The most important vector species belong to the genera: Anopheles, Culex, Aedes [5]. Human Malaria is caused by Plasmodium parasites transmitted by female mosquitoes, Anopheles. In West Africa, the most efficient vector is the Anopheles gambiae complex (Anopheles gambiae s. l.). The important freshwater species are An. gambiae s.s., An. arabiensis and the salt water breeding species, An. melas in the swamps [6,7]. Bancroftian filariasis in West Africa is caused by Wuchereria bancrofti and transmitted by Anopheles spp. in rural and Culex quinquefasciatus in urban areas [8,9]. In West Africa, Aedes aegypti is the vector of the causative virus of yellow fever [10] and vector of the causative virus of dengue fever in East Africa [11]. Although there had been progress in filariasis control since the initiation of Mass Drug Administration (MDA) programmes, challenges have emerged [12]. These challenges have led to growing concern on the effectiveness of MDA; involving vector control is now thought to have great potential to become an important supplementary component of the filariasis elimination campaign. Similarly, an Integrated Vector Management (IVM) has been recommended for malaria control by the World Bank [13], consisting of Long Lasting Insecticide Treated Nets (LLITNs), Indoor Residual Spray (IRS) and larviciding. An important prerequisite to larviciding, is an accurate knowledge of the breeding sites of major diseases vectors. The increasing number of students attending the University Health Centre for malaria and records of lymphatic filariasis at the adjacent University of Port Harcourt Teaching Hospital (Pers. Comm.) are of grave concern. Furthermore, the absence of LLITNs in students’ hostels and the volume of human traffic make IRS untenable. Hence, the focus of the IVM is on larviciding and this requires an accurate knowledge of breeding sites, which necessitated this study.
2. Materials and Methods
- Study AreaThe University of Port Harcourt is at the periphery of Port Harcourt metropolis, 4° 55′ 26 N and 6° 54′12 E, in lowland rain forest. There are two seasons: rainy (April-October) and dry (November-March). The heaviest precipitation is in September with an average rainfall of 367mm. December and January are usually the driest. Average temperature is 25-28°C [14]. The University consists of three parks: University (largest), Delta and Choba. Choba Park is gradually being converted to the commercial Park of the University and hence was not featured. Methods Collections of immatures were undertaken, 8.00-10.00hrs daily, 01 August- 30 September, 2018. Samples were collected weekly over a 6-week period from randomly selected probable breeding sites. A 100ml-silver ladle and Pasteur pipettes were used for immature collections. Immatures were identified by standard keys [5,15]. Larvae were reared to adults and identified by standard keys [16,17]. ANOVA (t-test, Correlation coefficient) and Post Hoc Tests were used for statistical Analyses.
3. Results
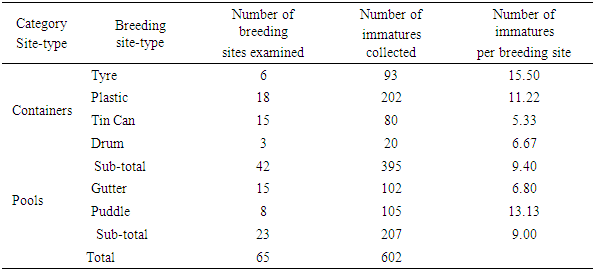
- Mosquitoes utilized both container and pool breeding site-types. Based on number of immatures per breeding site, the highest numbers were in tyres (container-type), puddle (pool-type) and plastic container, in descending order (Table 1). The lowest number per breeding site was from tin can. However, these differences were not significant (t>0.5). There was association among the 6 breeding site-types, because correlation coefficients between them were greater than 0.50.
|
|
4. Discussion
- Breeding sites of Culicidae were varied; diverse sites had also been recorded in other studies. Culex immatures had also been recorded from tyres, plastic and metal containers, pools [10,18,19,20]. Other studies recorded pit latrines as preferred breeding site types of Culex [9,19]. Anopheles immatures from containers (tyres, metal and plastic), borrow pit, hoof prints and pools had been reported [10,20,21,22]. Among container site-types, Rabiu and Ahmed [10] found discarded tyres most preferred at Ilorin, while Okorie [23] recorded container breeding site-types as least preferred at Ibadan, Nigeria. Aedes immatures were collected from containers (tyres, metal, plastic, pot) and pools, which conformed to results from earlier studies [10,20,24]. The diversity in breeding site-types from these results and those of the referenced studies, highlight the necessity for a thorough investigation of preferred breeding sites at each location, prior to larviciding. The high numbers of Anopheles, Culex, Aedes emphasize the need for integrated vector management, with specific focus on larviciding at the University, since species in these genera are vectors of the causative organisms of malaria, lymphatic filariasis and yellow fever [9,18,24]. The absence of a yellow fever epidemic in the area in the past decades could be attributed to the absence of the causative virus, although Mona monkeys (Cercopithecus mona) have been recorded in the area over the past 2 decades [24,25,26,27].
5. Conclusions
- The varying choices of breeding sites across locations highlight the need for detailed investigations at each locality to identify preferred sites prior to effective larviciding. The high numbers of immatures recorded in these studies stress the need for urgency in larviciding to reduce the incidence of malaria and lymphatic filariasis in the University.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML