-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Public Health Research
p-ISSN: 2167-7263 e-ISSN: 2167-7247
2017; 7(4): 91-99
doi:10.5923/j.phr.20170704.02

Prevalence and Antibiotic Susceptibility of Listeria monocytogenes in Retailed Meats in Port Harcourt Metropolis, Nigeria
Ngozi Nma Odu, Iheanyi Omezuruike Okonko
Department of Microbiology, University of Port Harcourt, Port Harcourt, Nigeria
Correspondence to: Iheanyi Omezuruike Okonko, Department of Microbiology, University of Port Harcourt, Port Harcourt, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

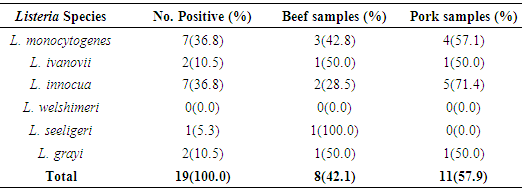
Listeria monocytogenes is the causative agent of Listeriosis, a disease of humans and animals owing to food and environmental contamination as well as zoonotic infections. Globally, this has become an emerging and zoonotic bacterial disease having low incidence with however high case fatality rate. The essence of this study was to isolate L. monocytogenes from retail beef and pork samples purchased from five markets within Port-Harcourt metropolis and to detect their antimicrobial profile. One hundred samples of retail beef and pork were purchased from different vendors across the five markets using cross-sectional study design from November 2013 to March 2014. The samples were examined for the occurrence of Listeria monocytogenes and other Listeria species using standard microbiological methods. From the study, of 100 samples (50 each for beef and pork) analyzed, 19(19.0%) confirmed the presence of Listeria species. Of these nineteen isolates, 7(36.8%) were Listeria monocytogenes while 12(63.2%) were other Listeria species [L. Ivanovic 2(10.5%), L. innocuous 7(36.8%), L. grayi 2(10.5%), and L. seeligeri 1(5.3%)] of the total samples. Although there was insignificant difference (p>0.05) in the contamination levels of beef and pork meat, there was however a substantial variation (p<0.05) in the contamination level of the different locations with Trans-Amadi and Creek road markets having the highest incidence of 7/19(36.8%) each, Mile-1 Market did not have any positive sample for Listeria. The antibiotics profile of the Listeria monocytogenes strains was done by using the standard disc diffusion method (Muller Hinton Agar) against 14 antibiotics. All isolates were (100.0%) susceptible to Chloramphenicol, Gentamicin, Ampiclox, Clotrimoxazole and Streptomycin. It also showed that they were all (100.0%) resistant to Augmentin, Erythromycin, Tetracycline, Rifampicin, and Cloxacillin. The isolates also showed varying degree of resistance to Norfloxacin (4 resistant, 57.2%), Levofloxacin (5 resistant, 71.4%), and Ciprofloxacin (5 resistant, 71.4%). This study further confirms the presence of Listeria monocytogenes and other Listeria species in beef and pork meat samples in Port Harcourt metropolis, Nigeria which is a cause for public health concerns because of the danger this poses especially through cross-contamination and inadequate cooking of meat products. There is a need for relevant public health agencies in Nigeria to create awareness to vendors and consumers about Listeria in food and its potential as a food pathogen of interest.
Keywords: Listeria monocytogenes, Listeria species, Antimicrobial drugs, Public Health
Cite this paper: Ngozi Nma Odu, Iheanyi Omezuruike Okonko, Prevalence and Antibiotic Susceptibility of Listeria monocytogenes in Retailed Meats in Port Harcourt Metropolis, Nigeria, Public Health Research, Vol. 7 No. 4, 2017, pp. 91-99. doi: 10.5923/j.phr.20170704.02.
Article Outline
1. Introduction
- Contaminated raw meats are one of the chief causes of foodborne infection [1, 2]. Campylobacter spp., Listeria monocytogenes and Escherichia coli O157: H7 were the major pathogens associated with meat and meat products. These organisms have been linked to a number of cases of human illnesses [3, 4]. L. monocytogenes is the causal agent of listeriosis, which is one of the most virulent foodborne diseases that controlling and monitoring agencies across the globe have been trying to contained [5].It is well established that listeriosis causes a range of manifestations including flu-like symptoms such as fever, fatigue, nausea, vomiting, and diarrhoea, and severe symptoms such as septicemia and meningitis [6]. Listeriosis has an approximately 30% case-fatality rate that increases to seventy-five percent in groups at high-risk which include pregnant women, fetuses, neonates, persons above 60years and immunocompromised adults [7, 8]. The obvious upsurge of contamination in food industry particularly poultry and meat products by pathogens has brought great public health concern. Both L. ivanovii and L. monocytogenes are pathogenic in mice, however, L. monocytogenes is solely and consistently related to human illness [9]. Listeria species has been isolated from poultry and red meat products in several countries such as Yugoslavia, Belgium, New Zealand, Australia and Japan [10]. The existence of Listeria species in meat calls for public health concerns in relations to the safety of consumers, as these Listeria species have the capability of developing on both cooked and raw meat during refrigeration [10]. Listeria species particularly L. monocytogenes has been related to an extensive diversity of food sources mainly chicken and red meat [11]. Genus Listeria contains ubiquitous bacteria that are extensively disseminated in normal environments. The ubiquitous trait of these pathogens unavoidably results in contamination of many foods and meat products [10]. Listeria species are resistant to harsh environments such as high salt environment, low temperatures and low pH [12, 13]. Consequently, they are present in a diversity of environments which include foods, water, soil, effluents, silage and sewage. Members of Listeria genera are extensive in the surroundings and spreading to animals. The results of their occurrences are advanced in the environment via milk, blood and faeces. In these conditions, food substances of the animal source are opened to contamination in a substantial degree that could happen in the course of transportation, processing and storage [14, 15]. Although milk and its products remained categorized to be mainly accountable in circumstances of listeriosis owing to food consumptions, researchers have revealed that L. monocytogenes can contaminate meat [16]. With globalization and intensified consumption of ready-to-eat foods globally, it is barely astonishing that L. monocytogenes has emerged as a vital foodborne pathogen of public health implication [17]. This study evaluated the occurrence of L. monocytogenes in retail meat and pork sold in different markets within Port Harcourt metropolis. It is necessary because of the significance of L. monocytogenes as an emerging pathogen of public health concern and considering the role and nutritional benefits of meat and pork as an essential part of human diet especially with the growing habit of eating half cooked meat (sushi meals) that is gradually being take in this part of the world. Hence, the need to study the prevalence and antibiotic resistance of L. monocytogenes isolated from beef and pork which are commonly consumed in a developing country like Nigeria. Thus, the aim of this study was to assess the prevalence of Listeria monocytogenes in beef and pork meat from selected markets in Port Harcourt metropolis, as well as the drug sensitivity pattern of L. monocytogenes, isolates from the sample area.
2. Material and Methods
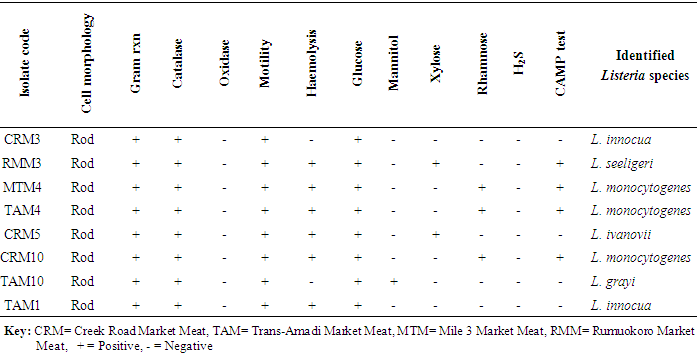
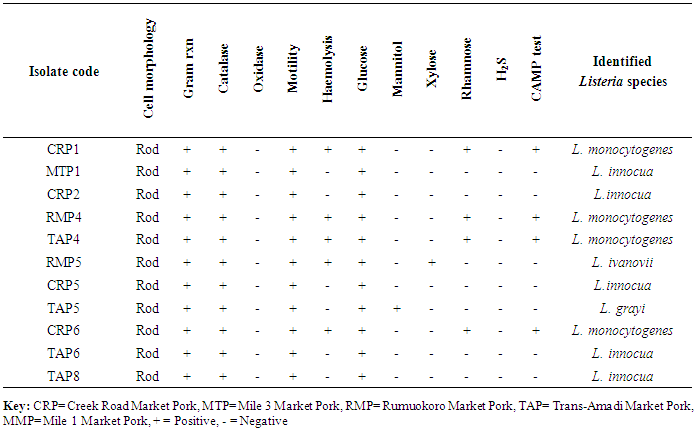
- Study areaThis study was conducted with beef and pork samples purchased at five markets viz Trans-Amadi Market, Rumuokoro Market, Creek Road Market, Mile 1 Market, and Mile 3 Market all within Port Harcourt metropolis. Samples were collected from five markets within Port Harcourt metropolis, they include Rumuokoro market (RM), Trans-Amadi slaughter market (TA), Mile 1 market (MM), Mile 3 market (MT) and Creek Road market (CR). The sampling was conducted between November 2013 and March 2014 during which samples were collected from the different markets periodically for analysis.Sample Collection and PreparationFifty beef and fifty pork meat samples were examined for the presence of Listeria monocytogenes. They were subjected to microbiological analysis to determine their microbial quality. Samples were collected from the five markets used as a study area and aseptically transported to the Laboratory in cold-boxes. Samples were kept at 4C and analyzed within 2 hours. The samples were given specific labels based on the location and type of meat (beef or pork). Trans-Amadi Market Meat (TMM), Trans-Amadi Market Pork (TMP), Rumuokoro Market Meat (RMM), Rumuokoro Market Pork (RMP), Mile-1 Market Meat (MMM), Mile-1 Market Pork (MMP), Mile-3 Market Meat (MTM), Mile-3 Market Pork (MTP), Creek Road Market Meat (CRM) and Creek Road Market Pork (CRP). All media were prepared and sterilized at 121C for 15 min in line with the manufacturer’s stipulation. Twenty-five grams of each sample with 225mls sterile 0.1% peptone water was placed in a stomacher bag for two minutes homogenization. A ten-fold serial dilution in sterile peptone water was prepared. Enumeration, Isolation and Identification of Listeria Monocytogenes and Listeria SpeciesThese were done according to the International Standards Organization (ISO 11290-1) methods of 1996 and 2004 [18, 19]. Briefly, Fraser broth (Oxoid, CM0895) was prepared and sterilized at 121C (15 lbs. psi) for 15 min in line with the manufacturer’s stipulation. Spread plate method was used in which 0.1ml of 10‾4-10-6 was spread plated in duplicates on the different media. The plates were incubated at 37C for 24h. Selective plating and characterization of Listeria colonies were carried out using Polymixin Acriflavine Lithium Chloride Ceftazidime Aesculine Mannitol (PALCAM) agar. The main discriminating enhancement step involves using a selective broth with reducing concentrations of Fraser broth. Also, 25g of meat samples were aseptically dispensed into a sterile stomacher bag enclosing 225ml of half Fraser broth (Oxoid CM0895, Basingstoke, United Kingdom) to attain a 1: 10 dilution factor [20, 21]. The mixture was vigorously mixed for 2min in the stomacher circulator (Unit 400, Seward, United Kingdom) and was incubated at 30°C for 24h. The second selective enhancement medium used is Fraser Broth (Oxoid, CM0895). After the incubation period, 0.1ml of pre-enriched cultures from half-Fraser broth culture was decanted into 10ml of Fraser broth for further enrichment. This was incubated at 37°C for 48h [20, 21]. A loopful of positive Fraser broth enriched culture was speckled onto PALCAM agar plates (Oxoid, CM0856) prepared and supplemented as directed. The plates were incubated at 37°C for 24 to 48h and thereafter observed for distinct colonies of Listeria. Proof of identity of Listeria colonies on PALCAM agar centred on their ability to hydrolyzed aesculin (blackening of the medium) and fermentation of mannitol (colour change from grey or red to yellow) owing to the production of acids [22] (Molla et al., 2004).Confirmation of Listeria monocytogenes and Listeria speciesDistinct colonies of Listeria were transferred onto pre-dried Tryptone Soya Agar (Oxoid, M290) plates complemented by 0.6 percent of Yeast Extract Powder ([TSYEA], Oxoid, LP0021) and incubated at 37°C for 24 hours after which further biochemical characterizations were carried out. Confirmation of Listeria isolates was based on motility, Gram reaction, catalase, and oxidase and further confirmed by their haemolytic ability on blood agar and their ability to ferment rhamnose mannitol and xylose sugar [23, 24]. Confirmation was also based on Christe Atkins Munch Peterson (CAMP) test according to ISO 11290-1 techniques [18, 19] (ISO, 1996, 2004).Antibiotic Susceptibility TestingAntibiotic sensitivity and resistance profile of L. monocytogenes isolate on Mueller Hinton Agar (MHA, Oxoid) were carried out using the disk diffusion technique. An inoculum from a cell suspension of approximately 106cells/ml was used for the test. The cell suspension was prepared by inoculating sterile normal saline with a pure culture of the test organism and incubating for 4hrs. Following this, the cell suspension turbidity was attuned to equal 0.5 McFarland Standard (0.5ml of 1% BaCl2 + 99.5ml of 1% H2SO4). To confirm the matching, the turbidities were also read through a spectrophotometer at 625nm prior to inoculation. The inoculum was applied onto Mueller-Hinton Agar (MHA, Oxoid) plates. These were left to dry at room temperature prior to aseptically application of the antibiotic discs and incubated at 37°C for 24h. A Clear zone of inhibition diameter was measured (in mm) and interpreted agreeing to the British Society for Antimicrobial Chemotherapy guidelines [25]. The activities of 14 antibiotics used in this study and the interpretations of the diameters of zone of inhibitions of the isolates were categorized as resistant, intermediate or susceptible in line with the recommendations by BSAC [25] standards. The abbreviations and concentration of the antibiotic discs (Oxoid, Australia) used were Ampiclox (APX) 20µg; Ciprofloxacin (CPX) 10µg; Chloramphenicol (CHL), 30µg; Rifampicin (RF) 5µg; Erythromycin (ERY) 5µg; Tetracycline (TET) 10µg; Gentamicin (GEN) 10µg; Amoxil (AML) 20µg; Augmentin (AUG) 30µg; Norfloxacin (NFX) 10µg; Levofloxacin (LEV) 20µg; Streptomycin (STR) 30µg; Clotrimoxazole (COT) 25µg; and Cloxacillin (CXC) 5µg.
3. Results
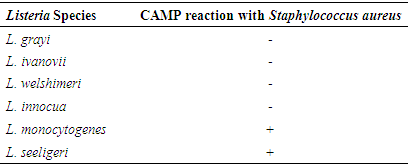
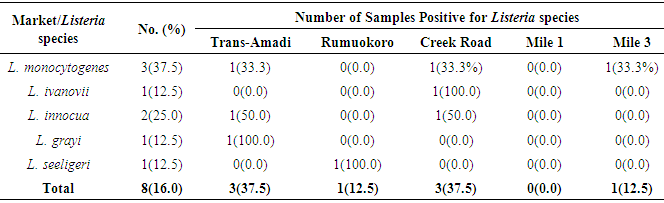
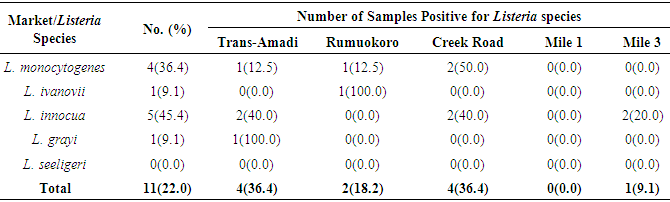
- CAMP testThe Christie-Atkins-Munch-Peterson test was used for confirmation of Listeria. All Listeria species were small colonies, positive for catalase and motile Gram-positive rods. They hydrolyze esculin and ferments dextrose sugar. Some of the species ferments rhamnose, mannitol and xylose with the release of acid. L. grayi ferments mannitol with the release of acid. L. monocytogenes and L. seeligeri induce hemolytic activities which were enriched in the zone predisposed by the Staphylococcus aureus streaked on sheep blood agar and were consequently CAMP test positive. However, L. monocytogenes does not utilize xylose and was positive for rhamnose utilization (Table 1).
|
|
|
|
|
|
|
4. Discussion
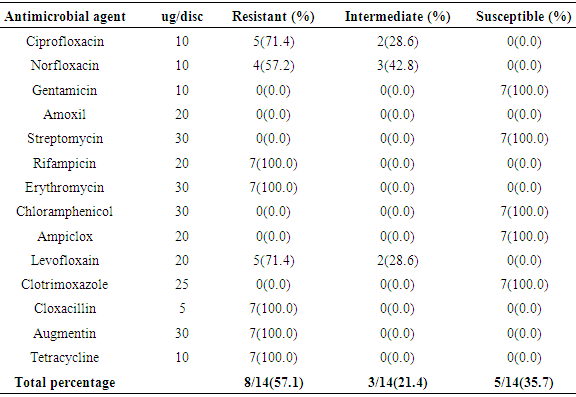
- In the present study, a total of hundred meat samples, fifty each of beef and pork across five different locations within Port-Harcourt metropolis were tested. Of the total 100 meat samples analyzed, 19(19.0%) had Listeria species, of which 7(36.8%) were Listeria monocytogenes while 12(63.2%) were other Listeria species [L. ivanovii 2(10.5%), L. innocua 7(36.8%), L. grayi 2(10.5%), and L. seeligeri 1(5.3%)]. The presence of L. monocytogenes in beef and pork meat samples is line with findings in previous studies and it has been reported in many countries, thus, underlining that contaminations may have occurred in the course of processing meat to the end and ready-to-eat products [26]. Of the 7(36.8%) Listeria monocytogenes isolated in this study, 3(42.8%) were found in beef meats and 4(57.1%) in pork meats samples. This result is in discordance with other studies regards to the presence of L. monocytogenes in foods. Eruteya et al. [27] recorded a rate of 1.29% of L. monocytogenes in raw cow and goat meat; Salihu et al. [28] reported 4.7% prevalence in beef. A number of authors reported a 4.65% and 6.4% (Bulgaria), 5.1% (Ethiopia), 6.66% (India), 17.7% (Portugal), 20.0% (Greek), 31.0% (Denmark) and 35.0% (Spain) prevalence of L. monocytogenes from raw beef. However, the high prevalence reported for L. monocytogenes in this study, 42.8% in beef meats and 57.1% in pork meats are in agreement with previous findings. Other authors had significantly higher percentage prevalence. Yusuf and Abdul-Hamid [29] recorded an incidence rate of 60.0% for L. monocytogenes in Kilishi, a meat product. Previous studies reported the prevalence of Listeria to be in the range of 0.0%-68.0% in pork meats [30-34]. The prevalence reported for L. monocytogenes in this study is higher than what was reported by some authors. Adetunji and Olaoye [35] also reported a rate of 20.0% in milk; while Nwachukwu et al. [36] recorded an even higher level in water samples; Gamboa et al. [37] reported 33.9% and Akya et al. [38] reported 27.2% in meat products. However, Okonko et al. [39] reported no occurrence of L. monocytogenes in kilishi. Okonkwo et al. [40] reported no occurrence of L. monocytogenes in raw vegetables and meat. Other previous studies in Nigeria, India, Serbia and Bangkok also reported the inability to isolate L. monocytogenes in beef and pork meat making it obvious that the occurrence rates of L. monocytogenes may vary from one place to the other depending on the methods used and also the predisposing conditions.The overall findings in this study coincide with that of previous studies which specified 1.0%-70.0% Listeria species prevalence in beef samples [22, 32, 34]. The custom of eating raw or undercooked meat (e.g. sushi) aggravates the public health risk related to L. monocytogenes [22]. The prevalence of L. monocytogenes in this study was higher in the pork meat samples with a prevalence rate of 8.0% than in beef meat samples (6.0%). It is also in disagreement with the work carried out by Molla et al. [22] in which an incidence rate of 7.5% was recorded in Pork meat and which was higher than all other meat samples. Lanciotti et al. [41] also observed the high occurrence of L. monocytogenes (17.6%) in pork in their study.The occurrence of these pathogens in swine is linked to the detail that swine are vectors of L. monocytogenes. For instance, it has been exposed that pork lodges L. monocytogenes in intestine and tonsils [42-44]. Owing to the facts, if there is rupture of the organ in the course of evisceration, the carcass can be contaminated with this pathogen. Even though it is recurrent for processing plants, slaughters and carcasses to be contaminated with L. monocytogenes, a subsequent daily wash of slaughtering slabs and sterilizing techniques ought to remove contamination. Meat products that are free of pathogens are contingent upon the efficiency of these sterilizing procedures [42].In this study, L. monocytogenes and L. innocua were most predominant with a prevalence rate of 7(36.8%) each, as compared with other species of Listeria. Thus, of all the Listeria species isolated, L. monocytogenes and L. innocua had an occurrence rate of 36.8% each, L. ivanovii and L. grayi, 10.5 % each while L. seeligeri had 5.3% occurrence of all the Listeria species. This is in agreement with other studies, Skovgaard and Morgen [45] showed that the occurrence of L. innocua as the dominating species in beef and poultry products. Also, Barros et al. [26] reported a higher prevalence of L. innocua in meat than all other Listeria species. Former studies showed L. innocua as the most predominant Listeria species observed in foods [22, 41]. Antibiotic susceptibility testing of the strains of L. monocytogenes isolated from beef and pork meats showed that an overall percentage resistance to be 57.1% and susceptibility to being 35.7%. All isolates (100.0%) were susceptible to chloramphenicol, gentamicin, ampiclox, cotrimoxazole and streptomycin. It also showed that they were all isolates (100.0%) resistant to augmentin, erythromycin, tetracycline, rifampicin, and cloxacillin. The isolates also showed varying degree of resistance to norfloxacin (4 resistant, 57.2%), levofloxacin (5 resistant, 71.4%), and ciprofloxacin (5 resistant, 71.4%). The high resistance of L. monocytogenes to these antibiotics could be owing to recent proliferation in the application of antibiotics as growth promoters [46]. Sometimes, antibiotics were added to animal feed for increased bulk of the animals.The 100.0% susceptibility of L. monocytogenes to gentamicin and ciprofloxacin as observed in our study was previously reported by Rahimi et al. [47] and Yakubu et al. [46]. They observed that more than 80.0% of L. monocytogenes were sensitive to these antibiotics. The 100.0% resistance of L. monocytogenes to erythromycin in the present study is in conflict with what was reported in Botswana where Morobe et al. [48] reported that all isolates in their study were sensitive to erythromycin. It is also in agreement with the study by Adetunji and Olaoye [35] in which 100.0% resistance was observed for augmentin and cloxacillin. High resistance observed for augmentin and cloxacillin (100.0%) shows that these widely used antibiotics will be unsuccessful in the treatment of Listeriosis. This was similarly observed in Ado-Ekiti where David and Odeyemi [49] reported that environmental isolates of L. monocytogenes were resistant to augmentin and cloxacillin. The observed resistance to antibiotics could be owing to selective antibiotic pressure [50]. The multi-drug resistance (MDR) observed in the present study has been previously observed by Lotfollahi et al. [51] and Yakubu et al. [46]. Yakubu et al. [46] in their study observed that 20.0% of isolates obtained from dairy foods showed resistance to two or more antibiotics. Yakubu et al. [46] also stated that none of the isolates in their study was resistant to less than one antibiotic. These observations are similar to what we observed in the present study with all isolates (100.0%) been resistant to greater than two antibiotics. This observation was also supported by Lotfollahi et al. [51] who found multi-drugs resistances to antibiotics in L. monocytogenes strains obtained from humans.
5. Conclusions
- This present study has further confirmed the existence of L. monocytogenes in beef and pork meat samples in Port Harcourt metropolis, Nigeria, which infers that likelihoods of acquiring listeriosis are significantly increased when undercooked meat and meat products are consumed. The presence of Listeria monocytogenes in meats is a cause for public health concerns because of the danger this poses especially through cross contamination and inadequate cooking of meat products. From the present finding, it can be inferred that Listeria monocytogenes is gradually becoming multi-drug resistant with emerging strains displaying multi-drug resistance (8 out of 14 drugs tested). There is a need for relevant public health agencies in Nigeria to create awareness to vendors and consumers about Listeria in food and its potential as a food pathogen of interest. In future, a sustained surveillance for emerging multi-drug resistance of this pathogen to antibiotics is highly essential. Also, this study advocates the essential for enhanced food safety by application of aseptic procedures at all stages of manufacture to consumption with specific highlights on ready-to-eat food substances which need no additional heat action.
ACKNOWLEDGEMENTS
- The authors sincerely acknowledge the assistance of Miss. Karimu Nimotalai in the collection and laboratory analysis of the samples.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML