Safaa Abu Mostafa1, Yousef Aljeesh2, Khitam Abu Hamad3, Mousa Alnahhal4
1Department of Neonatal Intensive Care Unit, Naser Medical Complex, Gaza Strip, Palestine
2Professor of International Public Health Medicine, Islamic University, Palestine
3Assistant Professor-School of Public Health, Al-Quds University, Palestine
4Head of Magnetic Resonance Imaging Department, European Gaza Hospital, Palestine
Correspondence to: Safaa Abu Mostafa, Department of Neonatal Intensive Care Unit, Naser Medical Complex, Gaza Strip, Palestine.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Neonatal hyperbilirubinemia is a widespread and significant clinical condition among neonates worldwide. This study aims at identifying the main risk factors, either socio-demographic, maternal or neonatal, that contribute to neonatal hyperbilirubinemia among hospitalized neonates in the Gaza Strip. The study is designed in a case-control fashion. The sample consists of 180 neonates (90 cases and 90 controls). Cases were selected from Al- Nasser pediatric hospital and Naser Medical Complex, while controls were selected from Khanyou ILS Martyrs clinic and Al-Remal Martyrs clinic. The researcher used an interview-structured questionnaire in the data collection process.Study results reveal that there is a significant association between family income and neonatal hyperbilirubinemia (P value < 0.05). Among maternal factors; bivariate test by person’s chi-square revealed that there were significant associations between hyperbilirubinemia and the mothers’ blood group, maternal anemia and pregnancy disorders (P value < 0.05). Concerning neonatal factors, bivariate analysis usinga person’s chi-square showed that birth weight and feeding practices that include feeding method, feeding initiation time, feeding difficulty, and feeding frequency were statistically significant risk factors for developing hyperbilirubinemia (P value < 0.05). Multiple logistic regression analysis were used to identify the main predictors of neonatal hyperbilirubinemia. The results showed that there was a statistically significant association between hyperbilirubinemia and family income groups; < 1800 ILS (AOR: 23.345, 95% CI: 2.083-261.688) and > 2300 ILS (reference group), maternal anemia groups; yes (AOR: 5.383, 95% CI: 1.035-27.998) and no (reference group), and birth weight groups; 2500 – 3000grams (AOR: 0.117, 95% CI: 0.028-0.498) and > 3000grams (reference group). All feeding practices shared a statistically significant association with hyperbilirubinemia occurrence, except the number of wet diapers/24hours as follows: feeding method groups; exclusive (AOR: 0.017, 95% CI: 0.003-0.093), bottle (AOR: 0.006, 95% CI: 0.000-0.141), and mixed (reference group), feeding initiation time groups; 1st hour (reference group) and more than 4 hours (AOR: 0.046, 95% CI: 0.004-0.586), feeding difficulty groups; yes (AOR: 0.079, 95% CI: 0.019-0.328), and no(reference group), lastly feeding frequency groups; on demand (reference group), every 2-3 hours (AOR: 0.108, 95% CI: 0.026-0.448)], and more than 3 hours (AOR: 0.003, 95% CI: 0.000-0.045). In conclusion, the study recommends the need for paying attention to ongoing screening and close monitoring ofat-high-risk neonates. In addition, emphasis should be directed at Health Education regarding effective feeding practices to reduce the rates of hospital readmissions and morbidities of neonatal hyperbilirubinemia.
Keywords:
Neonatal hyperbilirubinemia, Risk factors, Preterm, Neonatal Intensive Care Unit
Cite this paper: Safaa Abu Mostafa, Yousef Aljeesh, Khitam Abu Hamad, Mousa Alnahhal, Risk Factors of Hyperbilirubinemia among Admitted Neonates in the Gaza Strip: Case Control Study, Public Health Research, Vol. 7 No. 2, 2017, pp. 39-45. doi: 10.5923/j.phr.20170702.01.
1. Introduction
Neonatal hyperbilirubinaemia is the most common health problem among neonates; mainly in the first week of life. It occurs in about 60% of full-term neonates and in about 80% of preterm neonates; about 10% of breastfed babies show jaundice during the first month after birth (The Lancet, 2010). Features of neonatal jaundice include yellowish discoloration of the skin, sclera and mucous membranes resulting from accumulation of bilirubin in the skin and mucous membranes. This is associated with an increased level of bilirubin in the blood; a condition known as hyperbilirubinaemia (National Collaborating Centre for Women’s and Children’s Health, 2010). In specific terms, neonatal hyperbilirubinemia is defined as: a total serum bilirubin level above 5 mg per dL (86 μmol per L (AAP, 1994). In 2004, the American Academy of Pediatrics stated that hyperbilirubinemia in infants ≥35 weeks gestational age is defined as TB >95th percentile on the hour-specific Bhutaninomogram.Severe complication of neonatal hyperbilirubinemia may arise when bilirubin reaches toxic levels. This is referred-to as bilirubin encephalopathy or kernicterus. There is remarkable increase in the number of infants who develop kernicterus nationwide, which has made of the prevention of severe hyperbilirubinemia a National Priority (Cabra and Whitfield, 2005). The American Academy of Pediatrics (AAP) stated that jaundice has many possible risk factors, including blood group incompatibility (most commonly Rhesus or ABO incompatibility), maternal age ≥ 25 years, gestational diabetes, history of a sibling receiving phototherapy, cephalohematoma or significant bruising, exclusive breastfeeding, male gender, prematurity, and discharge from hospital after 72 hrs (AAP, 2004).The most common therapeutic modalities in the treatment of newborns with neonatal hyperbilirubinemia are phototherapy, exchange transfusion and intravenous immune globulin (Hansen, 2014).Globally, researches show that newborns who require phototherapy were estimated at 14.1 million babies annually, while approximately 100,000 reach extreme hyperbilirubinemia of Total Serum Bilirubin (TSB) 2 ≥ 30 mg/dL; a threshold associated with brain damage. The largest percentage is in low-income countries as in South Asia and Africa (Cline et al., 2011).Available evidence suggests that low and middle income countries represent the greatest burden of severe NNH characterized by high rates of morbidity, mortality and neurodevelopmental disorders compared to high-income countries (Olusanya et al., 2015). In sub-Saharan Africa, jaundice occurs in a high proportion of neonatal hospital admissions (Gordon et al., 2005). Olusanya and colleagues (2015) study illustrates that three-quarters of mortality occurred in sub-Saharan Africa and South Asia. In Cairo University Children‘s Hospital, (33%) of total admission cases to the out-born NICU in 2006 were diagnosed with severe neonatal hyperbilirubinemia (Iskander et al, 2012). Also, Yahya and Alajeely study (2013) in Mosul revealed that the incidence of neonates with hyperbilirubinemia reached (35%). Furthermore, Onyearugha et al. (2011) study revealed that NNJ accounted for 35% of all Neonatal Intensive Care Unit (NICU) admissions in Southeast Nigeria.Global estimatesin 2010 indicated that there were 114,000 neonatal deaths associated with Rh disease and bilirubin encephalopathy due to other causes (for 85/100,000 live births, 22% or 25,000 deaths with bilirubin encephalopathy). Eastern Europe/ Central Asia, Latin America, sub-Saharan Africa, and South Asia account for 6, 7, 35, and 39% of the deaths, respectively, with a combined prevalence of 119/100,000 live births compared with (0.1%) (n = 94; prevalence 1/100,000 live births) in high income countries (Bhutani et al., 2013). Although neonatal hyperbilirubinemia have been commonly studied worldwide, to the researcher best knowledge, this is the first research study to investigate risk factors of NNH in the Gaza Strip.
2. Methodology
The study is designed in a hospital-based and case-control manner; the study sample is a convenience sample which consists of 180 neonates (90 cases and 90 controls). Matching was done by age, gender and geographical area. The cases were all hospitalized newborns who were diagnosed with NNH and aged at 28 days or less, who were admitted in NICU of Al- Nassir pediatric hospital and Naser Medical Complex during the period between July and September 2015.The control group consisted of newborns aged from 29 to 49 days, didn‘t have NNH or history of other diseases and who presented for regular post-natal check-up at primary healthcare centers; either Khanyounis Martyers clinic and Al-Remal Martyers clinic in Gaza S trip. The data was collected using a well-structured questionnaire. The researcher analyzed the collected data using SPSS (version 20). Different statistical tests were used for data analysis including descriptive statistics, bivariate analysis using Chi-square and multiple logistic regression using Odds Ratio and confidence interval 95%.
3. Results and Discussion
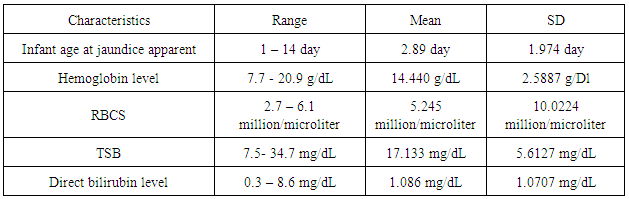
Descriptive analysis:Distribution of the characteristic of study casesThe case sample as shown in table (1), hemoglobin level ranged from 7.7 - 20.9 g/dL with a mean ± SD 14.440 ± 2.5887. RBCs count ranged from 2.7- 6.1 million /microliter with a mean ± SD 5.245 ± 10.0224.TSB ranged from 7.5 - 34.7 mg/dL with mean ± SD17.133 ±5.6127. Direct bilirubin level ranged from 0.3- 8.6 mg/dL with mean ± SD 1.086 ± 1.0707. First day jaundice was observed in 16 (8.9%) case. Thirty two (17.8%) of cases developed jaundice on the second day and 35(19.4%) between 3-5 days of life (table 4.4). Iskander et al. (2012) study showed that the mean age of jaundiced neonates was observed at 4.1 days of life, the mean age of presentation at the hospital was 9.4 days. In Olusanya et al. (2009) study, the median age of the jaundiced infants at the time of visiting the hospitals was 11 days.Table (1). Distribution of admission characteristics for the study cases
 |
| |
|
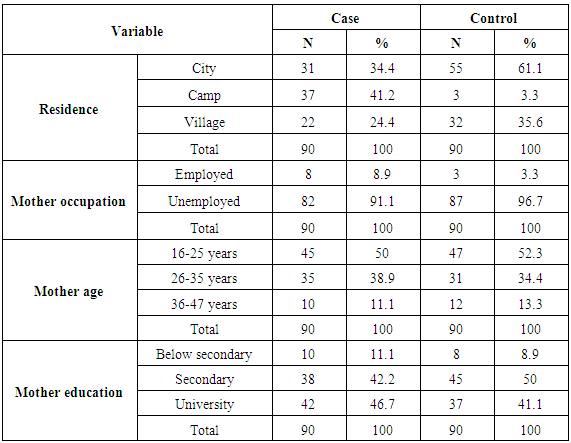
All cases in our study received phototherapy. Exchange transfusion was used in 9 (5%) of the cases; 2 cases in Naser Medical Complex and 7 cases in Al-Nassir Pediatric Hospital. Two cases showed signs of kernicterus and 2 cases had dysmorphic features (Down syndrome).The main cause for the development of NNH was ABO and Rh incompatibility (41.11%), followed by breastfeeding jaundice (23.33%) and in (15.56%) the cause was unknown. Dehydration was responsible for (7.78%), while sepsis accounted for (6.67%). There was a consensus between our results and international studies that the risk of developing NNH is higher in infants with ABO and Rh incompatibility. Henny-Harry and Trotman (2012) study found that the ABO incompatibility percentage was (35%) similarto Bhat and Kumar (2012) study which showed that ABO incompatibility was observed in (17.3%). Furthermore, Heydarian and Majdi (2010) study explained that the most common cause of exchange transfusion was ABO incompatibility (38.1%) and Rh incompatibility (16.1%). Moreover, Elhissi (2012) stated that physiological jaundice appeared in (28.1%) of newborns.Concerning the residential area, the table (2) shows that about (34.4%) of cases and (61.1%) of control are from cities, while (41.2%) of cases and (3.3%) of controlare from camps. In regard to village residents, about (24.4%) were cases and (35.6%) were controls.Table (2). Distribution of study participants byselected variables
 |
| |
|
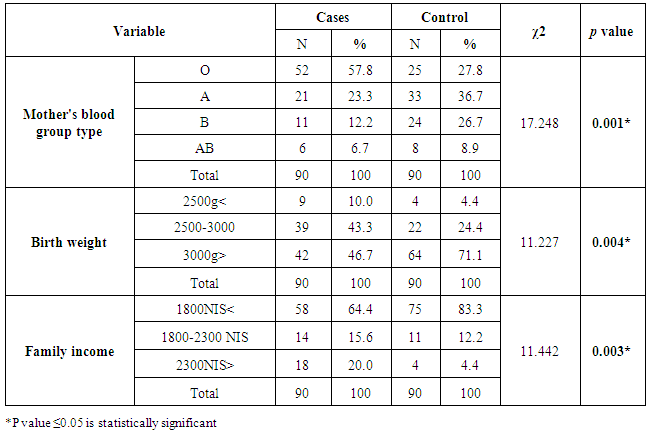
The mean age of mothers was 26.74 ± 6.188 years. The mothers’ ages of half of the cases (45) and 52.2% of controls (47) ranged between 16-25 years old. Also, 35 (38.9%) of cases’ mothers and 31 (34.4%) of controls’ mothersaged between 26-35 years. Only 10 (11.1%) of cases’ mothers and 12 (13.3%) of controls’ mothers belonged to the age group of 36 to 47. Moreover, study results indicate that 10 (11.1%) of cases’ mothers and 8 (8.9%) of controls’ mothers did not finish secondary school. Mothers of 38 cases (42.2%) and 37 controls (41.1%) were holders of a university degree. Regarding employment, the results show that the majority of controls and cases were children of unemployed mothers, at 87 (96.7%) and 82 (91.1%) respectively. While 8 (8.9%) of cases’ mothers and 3 (3.3%) of controls’ mothers were employed.For most of the study sample, (83.3%) of controls and (64.4%) of cases, income was less than 1800 ILS. Fourteen (15.6%) of cases and 11 (12.2%) of controls had incomes that ranged between 1800 to 2300ILS. The smallest group was made of 18 (20.0%) of cases and 4 (4.4%) of controls and had incomes more than 2300 ILS.Regarding the newborn’s birth weight, the results show that two groups of cases (46.7%) and (43.3%), weighted more than 3000grams and between 2500-3000grams, respectively. As for controls, (71.0%) and (24.4%) of controls, weighted were more than 3000grams and between 2500-3000grams, respectively. Overall, birth weight was in the range of 1800 – 4600grams. The mean was 3214.44 with SD ± 503.271. In Najib et al. (2013) study, birth weight ranged between 1550 – 4300grams and the mean was 3068 with SD (526) grams. Furthermore, Yahya and Alajeely (2013) study revealed that birth weight ranged between 1750 - 4800grams and the mean was 2.674, SD = 570.35.Among maternal factors; the results of bivariate test in table (3) revealed that there was a significant association between hyperbilirubinemia and the mother's blood group type; P value < 0.05. Birth weight as a neonatal factor demonstrated a significant association with neonatal hyperbilirubinemia, these results are consistent with Olusanya et al. (2015) study, in which underweight/weight loss was statistically associated with NNH (OR, 6.26; 95% CI: 1.23 - 31.86). Moreover, Friedman et al., (1978) study reported that neonatal jaundice and birth weight were significantly linked (P < 0.001). On the other hand, Kavehmanesh et al. (2008) study revealed that the mean (SD) of neonatal birth weight was 3301.1 (395) grams in jaundiced babies and 3303.5 (385.9) in non‐jaundiced babies] and there was no statistically significant association between groups (P value = 0.41). In regard to family income, there was a statistically significant association between family income and NNH (χ2 = 11.442, P value = 0.003). These results are incongruent with Olusanya et al. (2015) meta-analysis study which examined the role of social class on severe hyperbilirubinemia because the results showed no significant association (P value = 0.090). Also, Ng and Chong (2014) study verified that the mean family income was not associated with an increased risk of severe hyperbilirubinemia (P value = 0.682).Table (3). Bivariate analysis of risk factors of Neonatal Hyperbilirubinemia by selected variables
 |
| |
|
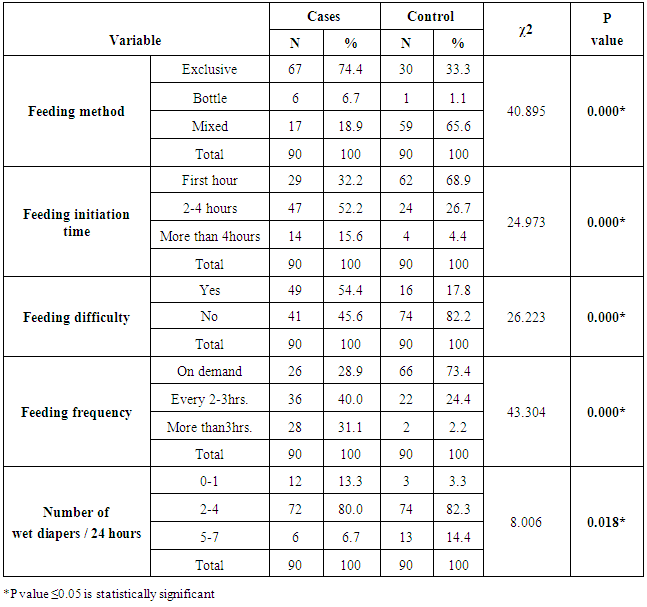
Concerning neonatal factors; bivariate analysis showed that birth weight, and feeding practices; including feeding method, feeding initiation time, feeding difficulty, feeding frequency, and number of wet diapers/24 hours, were statistically significant risk factors for developing hyperbilirubinemia as P value was < 0.05. Other factors such as newborn order, cephalohemtoma and bruising, and history of a sibling with jaundice were statistically all insignificant factors in the development of hyperbilirubinemia as P values were> 0.05. Previous studies had findings similar to ours. Heydarian and Majdi (2010) study explained that the majority of jaundiced neonates (57.6%) were exclusively breastfed, (26.3%) fed on both breast milk and formula, and (11%) took formula alone, (P value = 0.000). Moreover, Saigal et al. (1982) study showed that mean TSB were significantly higher in breast-fed than formula-fed infants on each postnatal day (P value < 0.001). Also, Najib et al. (2013) study revealed that the time of first feeding of jaundiced cases was 3.99 (9.99) hours after birth (Min: 0.5 hour, Max: 72 hours). Thirty three neonates (18.8%) had poor feeding, irritability and lethargy on admission and 4.65(6.58) hours after birth (Min: 1hr, Max: 24 hours). Table (4). Association between feeding practices and neonatal hyperbilirubinemia
 |
| |
|
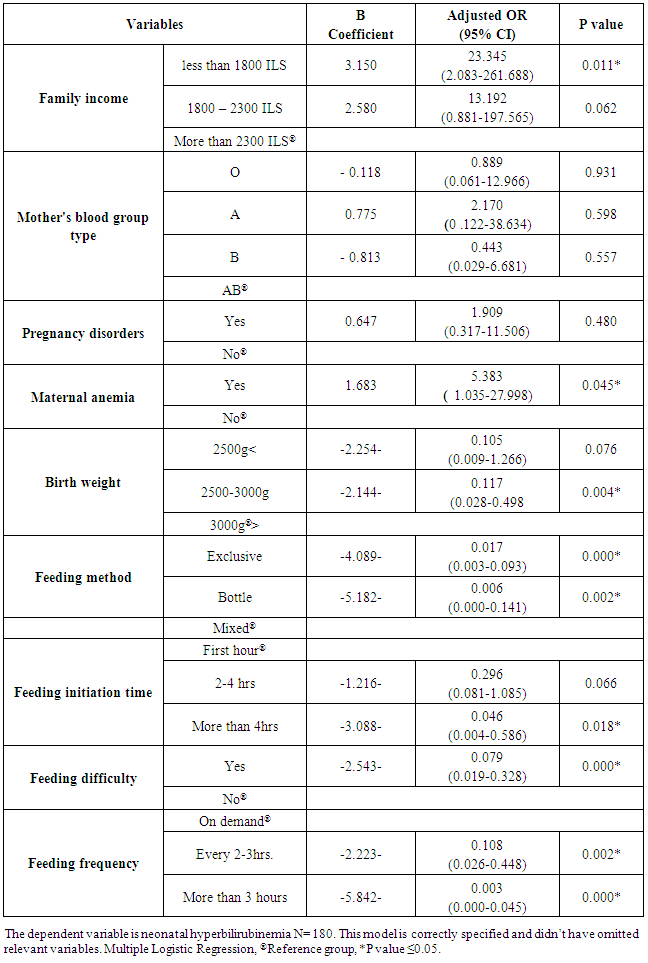
On the other hand, Bertini et al. (2001) study demonstrated a statistically significant positive correlation between patients with a TSB >12.9 mg/dL and supplementary feeding [refers to infants who were breastfed and received additional formula supplements.] (13.1%), (P value < 0.001) with mean 478 (22%); while, breastfeeding showed negative correlation in patients with a total serum bilirubin concentration >12.9 mg/dL (221 mmol/L) (2.7%), (P value < 0.001) with mean 1595 (73.4%).Table (5) shows the final model of risk factors for NNH after adjusting the odd ratio. The results shows that newborns who come from families of income more than 2300 New Israeli Shekel (ILS) have 13.192 times increased odds of developing NNH while those who come from families of income between 1800 and 2300 ILS have a marginally significant risk. Newborns who had families with more than 2300 ILS have 23.345 times increased odds of having NNH than families who had less than 1800 ILS, this is statistically significant. Table (5). Multivariate logistic regression of neonatal hyperbilirubinemia in Gaza Strip
 |
| |
|
The probability of NNH occurrence among neonates whom mothers hadn't anemia was 5.383 times than neonates of anemic mothers; this proved to be statistically significant. It implies that infants of mothers who had no pregnancy disorders have higher risk for NNH development. Newborns who weigh less than 2500grams have lower odds (0.105) of having NNH than newborns with birth weight of more than 3000grams. It is statistically significant that newborns of birth weights between 2500-3000grams show lower odds (0.117) of developing NNH than those with birth weights of more than 3000grams. This means that the probability of NNH is higher in neonates weighing less than 3000grams. In comparison to neonates who had mixed feeding, those who had exclusive breastfeeding or bottle feeding have decreased odds of having NNH by 0.017 and 0.006 times; respectively. Furthermore, newborns who had feeding difficulties have decreased odds of having NNH by 0.079 times than who didn't. This represents a positive association between feeding difficulties and the increased risk for NNH. The probability of NNH development was significantly lower among neonates who had their feeding on demand compared to neonates who fed every 2-3 hours (OR 0.108). This means that infants who are fed on demand had lower risk (negative association) for development NNH. The probability of NNH development was significantly lower among neonates who initiated their feeding within 1 hour of delivery. The odds of NNH occurrence were 0.046. In other words, there was a negative (protective) association between early initiation of feeding and the risk of frequency of NNH occurrence. On the other hand, mother's blood group type, pregnancy disorders and number of wet diapers/24 hours were not significant predictors of development NNH development as shown in table (4). Our results were consistent with others in international literature. Hung (2004) study showed that breast feeding was a statistically significant factor related to NNH based on the univariate logistic regression models [AOR: 4.60. 95% CI:2.40 - 8.81; (P > 0.001)] and similarly, multivariate logistic regression models adjusted for the breast feeding confirmed the statistical significance of this factor [AOR: 4.64. 95% CI: 2.25–9.57; (P > 0.001)]. Additionally, Kuzniewicz et al. (2008) study demonstrated in their multivariate model that infants who were exclusively breast-fed after their qualifying TSB had an AOR of 2.03 (95% CI = 1.03 to 3.99).
4. Conclusions and Recommendations
The study concludes that there is a significant association between hyperbilirubinemia and family income, mother's blood group type, maternal anemia, birth weight and feeding practices (feeding method, feeding initiation time, feeding difficulty, feeding frequency, and number of wet diapers /24 hours) since the P value < 0.05. In Gaza Strip, there is an urgent need to enhance the use of the evidence-based risk assessment chart for all delivered neonates before their discharge from delivery rooms. Moreover, there is a need to effectively monitor TSB for all delivered neonates before discharge in order to identify infants at risk of neonatal hyperbilirubinemia. Finally, the Ministry of Health should implement strong measures to encourage effective feeding practices and should adopt programs that aim to enhance neonatal feeding practices among mothers.
References
| [1] | American Academy of Pediatrics (1994). "Practice parameter: management of hyperbilirubinemia in the healthy term newborn". Pediatrics, 944(1), 558–562. |
| [2] | Bhutani, V., Zipursky, A., Blencowe, H., Khanna, R., Sgro, M., Ebbesen, F., Bell, J., Mori, R., Slusher, T., Fahmy, N., Paul, V., Du, L., Okolo, A., Almeida, M., Olusanya, B., Kumar, P., Cousens, S. & Lawn., J. (2013). "Neonatal hyperbilirubinemia and Rhesus disease of the newborn: incidence and impairment estimates for 2010 at regional and global levels". Pediatric Research, 74(1), 86-100. |
| [3] | Cabra, M. & Whitfield J. (2005). "The challenge of preventing neonatal bilirubin encephalopathy: a new nursing protocol in the well newborn nursery". Baylor University Medical Center Proceedings, 18(3), 217–219. |
| [4] | Cline, K., Vilms, R., Mcgraw, K., Lou, H., Donaldson, M. & Bhtani, K. (2011). "Global Burden and Unmet Need for Hyperbilirubinemia Treatment". Poster presented at: Pediatric Academic Societies; Available from: https://drev.s3uswest2.amazonaws.com/assetsold/pdf/2011_PAS_Global_Unmet_ Need_for_Phototherapy.pdf. [Accessed at: April 30-May 3 2011]. Denver, CO. |
| [5] | Gordon A, English M, Tumaini Dzombo J, Karisa M. & Newton C. (2005). "Neurological and developmental outcome of neonatal jaundice and sepsis in rural Kenya". Tropical Medicine and International Health, 10(11), 1114-1120. |
| [6] | Hansen, T. (2014). "Neonatal jaundice". Medscape. Available from: http://emedicine.medscape.com/article/974786-overview#showall [Accessed at: 4 April 2015]. |
| [7] | Hung M., Kua K., Teng H., Tang K., Weng H. & Huang C. (2004). "Risk Factors for Severe Hyperbilirubinemia in Neonates". International Pediatric Research Foundation, 56(5), 682-689. |
| [8] | Iskander, I., Gamaleldin, R. & Kabbani, M. (2012). "Root causes for late presentation of severe neonatal hyperbilirubinaemia in Egypt". Eastern Mediterranean Health Journal, 18(8), 882-887. |
| [9] | Kuzniewicz, M., Escobar, G., Wi, S., Liljestrand, P., McCulloch & Newman, T. (2008). "Risk factors for severe hyperbilirubinemia among infants with borderline bilirubin levels: A Nested Case-Control Study". Journal of Pediatrics. 153 (2), 234–240. |
| [10] | National Collaborating Centre for Women‘s and Children‘s Health (2010). "Neonatal Jaundice": Clinical Guideline. Royal College of Obstetricians and Gynecologists, London. |
| [11] | National Collaborating Centre for Women‘s and Children‘s Health (2010). "Neonatal Jaundice": Clinical Guideline. Royal College of Obstetricians and Gynecologists, London. |
| [12] | Olusanya, B., Osibanjo, F. & Slusher, T. (2015). "Risk Factors for Severe Neonatal Hyperbilirubinemia in Low and Middle-Income Countries": A Systematic Review and Meta-Analysis. PLoS ONE, 10(2), 1-16. |
| [13] | Onyearugha, C., Onyire, B., &Ugboma, H. (2011)."Neonatal jaundice: Prevalence and associated factors as seen in Federal Medical Centre Abakaliki, Southeast Nigeria". Journal of Clinical Medicine and Research, 3(3), 40-45. |
| [14] | Yahya, B., & Alajeely, S. (2013). "Incidence and risk factors of hyperbilirubinemia in neonatal in Mosul City". Kufa Journal for Nursing Sciences, 3(1), 39-49. |



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML



