-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Public Health Research
p-ISSN: 2167-7263 e-ISSN: 2167-7247
2017; 7(1): 27-34
doi:10.5923/j.phr.20170701.03

High Seronegativity of Immunoglobulin G (IgG) Antibodies against HPV in Pregnant Women in Ibadan, Nigeria
Okonko IO, Egbunefu NN
Medical Microbiology Unit, Department of Microbiology, University of Port Harcourt, Port Harcourt, Nigeria
Correspondence to: Okonko IO, Medical Microbiology Unit, Department of Microbiology, University of Port Harcourt, Port Harcourt, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The study determined the seroprevalence of anti-HPV-specific IgG antibodies in pregnant women. It also assesses immune status of pregnant women to existing HPV VLP vaccines and their vulnerability to the viruses. Ninety-one consented pregnant women (age range 18-43 years) were successively enlisted to the study. Relevant socio-demographical characteristics of the paticipants were collected with a questionnaire specifically designed for the study. Five milliliters of blood sample was aseptically collected from each pregnant woman and tested for antibodies to HPV with HPV IgG ELISA kits. All the women were sexually active being pregnant and in the 18-43 years age group. Eighty-seven (95.6%) of them responded ‘no’ to HPV vaccination. The 4.4% of the pregnant women that reported HPV vaccination also had no detectable anti-HPV antibodies. It showed that 4.4% of vaccinated women had not seroconverted at sampling time or did not immunologically respond to the vaccine. The study revealed 100.0% anti-HPV antibody negativity. Though, the 18-25, 26-35 and 36-43 years age-groups varied significantly. The 100.0% seronegativity among the pregnant women indicates a 100.0% vulnerability to infections with the four HPV genotypes.
Keywords: Antibodies, ELISA, HPV, IgG, Pregnant women, Seronegativity
Cite this paper: Okonko IO, Egbunefu NN, High Seronegativity of Immunoglobulin G (IgG) Antibodies against HPV in Pregnant Women in Ibadan, Nigeria, Public Health Research, Vol. 7 No. 1, 2017, pp. 27-34. doi: 10.5923/j.phr.20170701.03.
1. Introduction
- Human papillomaviruses (HPVs) are the utmost common and prevalent sexually-transmitted infectious agents and causative agents of cervical cancer (CC) [1-3]. Currently, 120 diverse HPV genotypes, strains or subtypes have been categorized to infect human [4]. About 30 of these are sexually-transmitted and are primarily transmitted to the genital tract through sexual contact. HPV subtypes were broadly divided into two groups low-risk HPV (lrHPV) and high-risk HPV (hrHPV) according to their degree of risk of development of cancer after infection and their epidemiological relationship with cervical cancer [1, 2]. The lrHPV group includes 6 and 11 subtypes and is believed to be connected with recurrent respiratory papillomatosis, benign lesions or genital warts [1-2, 5]. The hrHPV group includes 16 and 18 subtypes and triggers precancerous cervix lesions in 5 to 10% of infected women. Precancerous cervix lessions includes cervical intraepithelial neoplasia, which can progress to invasive cancer of the cervix or ano-genital cancer 15–20 years later [1, 2, 6].Infection of the genitals with hr HPVs is actually occurring with peak prevalence in women under 30 years old [7-14]. It has been widely reported that persistent infection with hrHPV subtypes is the main cause of cancer of the cervix [1-3]. Fortunately, HPV infection is momentary in 90% of women who are immunocompetent [15-17]. The HPV prevalence is projected at 21.1% in Africa, having the Sub-Saharan region topmost in listing with 24% prevalence [2, 18, 19]. The prevalence of HPV is high in all female age groups, and highest among women within 15–23 years old in Nigeria [2, 19, 20]. In a previous study in Nigeria, 26.3% HPV prevalence has been reported in the general population in Southern Nigeria [2, 20, 21]. Also in Nigeria, the incidence of HPV was reported to be 24.8% in women with cancer of the cervix [2, 19, 22] and 23.7% among women having normal cytology [2, 11]. Also, Aminu et al. [2] reported a 42.9% prevalence for HPV-IgG antibodies in women in Kaduna State, Nigeria, signifying that these women were infected previously and that the virus is still circulating with a high prevalence [2].Bivalent (HPV16/18) or quadrivalent (HPV6/11/16/18) virus-like particle (VLP) vaccines have been approved for human use [10-14, 23]. These vaccines comprises major L1 structural proteins of the oncogenic and/or non-oncogenic HPV subtypes. These vaccines have presented more than 90% efficacy and 100% immunogenicity against persistent infections of HPV16/18 subtypes in developed countries where it has been used [10-14, 24, 25]. The endorsement of HPV vaccination in girls and young women is founded on “immunological bridging”, i.e., the demonstration of higher or similar antibody production/secretion in women whose clinical efficacy against cervical cancer in situ was revealed [3, 26]. Also, the period of vaccine defense and the necessity of a booster dosage will be established partially on serological investigation of antibodies to HPV [3].Furthermore, seroepidemiology have been very valuable in understanding the natural account of HPV and in assessing HPV contact in the population to ascertain target population for HPV immunization schedules [3]. HPV antibodies are stable over five years [27] and they are suitable immunological markers for cumulative exposure (past and current) to HPV infections. The methods successfully used in HPV serology are VLP-based ELISA, pseudovirion-based neutralisation assay, Luminex assays, and Multiplex serology assays.Whereas presence of HPV DNA indicates recent infection of the cervix, occurrence or persistency, however, it is not an indicator for increasing contact to HPV [3, 28]. Nonetheless, increasing HPV exposure is best ascertained by measuring HPV-specific IgG antibodies [3, 29, 30]. Serum IgG are viewed as the identifiers/markers of infections that ensued throughout the lifespan of the persons since they continue after DNA turn out to be undetectable [3, 29, 30]. Though HPV serology is an imperfect indicator of previous contact since nearly half of the women with DNA-positivity are seronegative [3, 31, 32], nowadays it is seen as an indicator of increasing HPV infection [3, 33]. In addition, the serum antibody titer measured by VLP-based enzyme immunoassay is highly correlated with the neutralizing antibody titer, thereby indicating that VLP-based enzyme immunoassay can serve as a surrogate marker for neutralizing antibodies [34, 35]. The VLP ELISAs generally detect only one immunoglobulin subclass (IgG, M or A), dependent on the specificity of the detecting antibody [36]. Therefore, determining the susceptibility and exposure of humans to HPVs would rely on detection of HPV type-specific antibodies, and the VLP ELISA has been used in achieving this [37, 38]. VLP-based ELISAs have been used in a number of epidemiological studies [39, 40].To establish efficiency of HPV vaccination programmes and the cumulative exposure to HPV, it becomes essential to determine the seroprevalence of anti-HPV-specific IgG antibodies in pregnant women. The study also assesses immune status of pregnant women to existing HPV VLP vaccines and their vulnerability to the viruses.
2. Methods
- Study areaPregnant women in Adeoyo Maternity Teaching Hospital, Yemetu, Ibadan; St. Mary Specialist Hospital, Eleta, Ibadan and University College Hospital, Ibadan, Nigeria were recruited for the study. These study areas are situated at Ibadan municipal areas. Ibadan, the Oyo State capital is situated in the forest zone of South West region of Nigeria. In addition to being the biggest native city in south Saharan Africa, the city is a significant educational and trade centre. It also the home of one of the largest and leading teaching hospitals in Africa. Study populationsThe study sample comprised 91 pregnant women recruited from June, 2013 to March, 2014; their age range was 18 – 43 years. Samples and pertinent data were collected. Ninety-one consented pregnant women (age range 18-43 years) were successively enlisted to the study. Relevant socio-demographical characteristics of the participants were collected with a questionnaire specifically designed for the study (Tables 1 and 2). Blood Sample collection, serum preparation and storageThree to five mililitre (3-5 ml) blood sample was collected aseptically from each pregnant woman and decanted into properly labeled blood sample tube with no anticoagulants, and kept at room temperature for 40 min, afterwards spun at 3,000 rpm for 10 min. The resultant sera was stored in an Eppendorf tubes at -20°C until used for serologic testing. Samples were conveyed to the Medical Microbiology Unit, Department of Microbiology, University of Port Harcourt, Nigeria in a cold chain. Methods were in agreement with the ethical standards of the Nigerian National Code for Health Research Ethics and the Declaration of Helsinki (October 2008 revision) as issued by Oyo State Ministry of Health AD13/479/349. Serologic testingSera were analysed for the presence of anti-HPV-specific IgG antibodies against four HPV subtypes (6/11/16/18) in vitro using a commercially available HPV-IgG kit (DIA.PRO Diagnostic Bioprobes, Milano, Italy) based on Enzyme-linked Immunosorbent Assay (ELISA) as described previously [37, 38, 40, 41]. The serologic test and interpretation of results were done according to instructions of the kit manufacturer. Optical signals generated in the microwells were read at 450 nm with an ELISA plate reader (BioTek Instruments®, Model ELx800; BioTek, Highland Park, Winooski, U.S.A). The ELISA kit manufacturer provided the formula for calculating the cut-off OD450nm (OD of negative control plus 0.250) which we used as threshold for determining the reactive and non-reactive serum samples. Principle of the TestMicroplates are coated with recombinant VLP’s derived from HPV Type 6, 11, 16e 18. In the first incubation, the solid phase is treated with diluted samples and anti-HPV IgG are captured, if present, by antigens. After washing out all the other components of the sample, in the 2nd incubation bound anti-HPV IgG are detected by the addition of anti IgG antibody, labeled with peroxides (HRP). The enzyme captured on the solid phase, acting on the substrate/chromogen mixture, generates an optical signal that is proportional to the amount of anti-HPV IgG antibodies present in the sample. A cut-off value turns the measured optical densities into positive or negative results.Assay procedures1) The samples were diluted by dispensing 10µl sample and 1ml specimen diluent into a dilution tube (1:101 dilution factor), and then mixed properly. 2) The required number of microwells was placed in the microwell holder. 3) The well in position A1 was left empty for blanking. 4) A 100µl of Negative Control and 100µl of Positive Control were dispensed in the proper wells in duplicate. 5) Then, 100µl diluted samples were dispensed in the appropriate wells. 6) The microplate was incubated for 60 minutes at 37°C. 7) The strips were sealed with the supplied adhesive sealing foil. 8) After incubation, the microplate was washed using the calibrated ELISA microplate washer (BioTek Instruments®, Model ELx50; BioTek, Highland Park, Winooski, U.S.A) by delivering and aspirating. 9) A 100µl of the Enzyme Conjugate was pipetted into each well, except the blanking well A1 and covered with the sealer. All the wells were ensured to be red coloured. 10) The microplate was incubated for 60 minutes at 37°C after which it was washed as described in step 8. 11) A 100µl of the chromogen/ substrate mixture was pipetted into each well, the blank well A1 included. 12) The microplate was then incubated at room temperature for 20 minutes. Microplates were not exposed to strong direct illumination to prevent generating high background. 13) A 100µl of sulphuric acid was pipetted into all the wells including the blank well. The addition of sulphuric acid turned the positive control and positive samples from blue to yellow. 14) The colour intensity of the solution in each well was measured according to the manufacturer’s specifications at 450nm filter (reading) and at 630nm (background subtraction), blanking the instrument on Al.Cut-Off CalculationIf data are valid, the mean OD450nm value of the Negative Control (or NC) was calculated and then the following formulation was applied to calculate the cut-off value: NC + 0.250 = Cut-Off. Interpretation of resultsSamples with an OD450nm lower than the Cut-off value were considered not reactive for IgG specific to the HPV antigens present in the vaccine. Samples with an OD450nm higher than the Cut-Off value were considered positive for IgG specific to the HPV antigens present in the vaccine. In the cases the quantification of IgG present in positive samples is required to better monitor the immunological responses to the vaccine in a prolonged time, OD Sample/Cut-Off (or S/Co) value was calculated for each sample and that provided an index whose value is directly proportional to the content of IgG in the sample.
3. Results
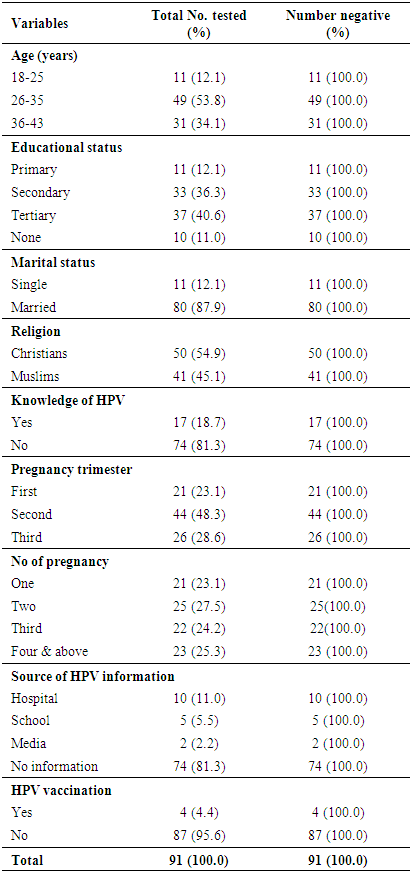
- Patient characteristics The age-range of ninety-one pregnant women was 18-43 years (Table 1).
|
4. Discussion
- The aim of this present study is to ascertain the seroprevalence of anti-HPV-6/11/16/18 specific-IgG antibodies in pregnant women. It also assesses immune status of pregnant women to existing HPV-VLP vaccines and their vulnerability to the viruses. In a study by Moscicki [42], about half of the women who had no HPV infection became HPV-infected within 3 years afterward starting sexual activity [1]. The occurrence of lrHPV might reveal a form of novel HPV infections, since the clearance rate for lrHPV was higher compared to hrHPV which had a shorter infection duration [1, 43]. HPV seroprevalence does not show the present state of HPV infection owing to a lower seroconversion rate after infection [1]. However, it is believed to be a valuable indicator of increasing HPV contact [1].HPV seropositivity has been shown to be a biomarker of past and present HPV infection and lifetime number of sexual partners [44, 45]. Evaluating the vulnerability and contact of human beings to HPV infections would be dependent on detecting HPV subtype-specific antibodies, and virus-like particle ELISA has been utilized in accomplishing this [37, 38]. In the present study, we could not demonstrated seropositivity to any of the common HPV subtypes (6/11/16/18) amongst pregnant women; this is similar to the findings of earlier studies [38, 41], however, some studies outside Nigeria established HPV seropositivity [39, 40]. The zero HPV seropositivity found in these pregnant women studied is consistent with earlier studies which reported low HPV prevalences in Sub-Saharan African women. The 0.0% serpositivity of HPV in this study is lower compared to the 3.8% in Zaria, Nigeria [46]; the 4.9% in Port Harcourt, Nigeria [37]; the 6.6% in Ilesha, Nigeria [38], and the 42.9% in Zaria, Nigeria [2]. However, this zero HPV seropositivity is inconsistent with earlier studies in Sub-Saharan Africa with elevated HPV prevalences [47-50], signifying that these women may not have had any previous exposure or that the virus is not circulating with a high prevalence. This deviate from what was reported by Aminu et al. [2].The 100.0% observed among these pregnant women evaluated might be owing to the detail that these pregnant women were unexposed to any of the lrHPV and hrHPV subtypes or they must have had untraceable antibody production/secretion at the period of sampling. It could also be owing to characteristic sensitivity of the ELISA kit used. Therefore, the high general seronegativity rate reported for anti-HPV-6/11/16/18 specific IgG-specific antibodies in this study suggests that the seronegative pregnant women are vulnerable to HPV infection [38, 41, 51]. Globally, the seroprevalence of HPV differs with regional variations, age, marital and educational status [1]. In this study, the age-range of ninety-one pregnant women was 18-43 years. This is also comparable to the age-ranges in earlier studies [2, 38, 41]. Age group 26-35 had the highest frequency (53.8%), followed by age group 36-43 years (34.1%). Age group 18-25 (12.1%) was the least and comparisons of the three age-groups. Earlier studies have stated that seroprevalence of HPV is linked with marital status, age at sexual debut, past history of STD, tribe, number of sex partners, religion and educational background [1, 2, 52-54]. The pregnant women generally had high level of education (40.6% had tertiary education and 33.6% had secondary education) with 87.9% being married. About 54.9% of the pregnant women were Christians and 45.1% were Muslims.In regard to behavioral risk factors in this study, not any of these revealed statistical connotation with seronegative outcomes. Group-specific seronegative outcomes were in equal proportion (100.0%) for pregnant women in at all levels. This is comparable to earlier findings by Aminu et al. [2] and Okonko et al. [41]. In addition, the zero seropositivity for anti-HPV-6/11/16/18 specific IgG antibodies amongst pregnant women is alarming notwithstanding that majority of the pregnant women had tertiary educational level. This deviated from earlier finding in Nigeria [21, 55, 56]. It also differed from earlier finding by Thomas et al. [50] who stated that uneducated women in Ibadan had elevated HPV seropositivity.Majority (81.3%) of the women were ignorant of HPV infections and its consequences while 18.7% were aware of HPV infection. Of the 17 (18.7%) pregnant women who were aware of HPV, 10 (11.0%) got their information from hospital, 5 (5.5%) from school and 2 (2.2%) from the media. Forty-four pregnant women (48.3%) were in their second trimester of pregnancy, 26 (28.6%) in their third trimester while 24 (23.1%) were in first trimester. Of all the pregnant women tested, 25 (27.5%) were having their second pregnancy, 25.3% have had more than three pregnancies, 24.2% were having their third pregnancy and 23.1% had their first pregnancy. Majority (95.6%, n=87) of the pregnant women had never been vaccinated against HPV while only 4(4.4%) were vaccinated against HPV. It is striking that notwithstanding having 4(4.4%) pregnant women who received HPV vaccination, they still were seronegative to anti-HPV-6/11/16/18 specific IgG antibodies. This seronegativity status might be owing to the fact that their serum anti-HPV-specific IgG antibodies were below measureable levels or the pregnant women had not seroconverted at the period when their samples were collected [38]. However, HPV re-vaccination is advocated [38, 41]. A statewide awareness/education campaign and HPV vaccination of all women of reproductive age as well as sexually energetic females is also suggested. Limitation of the present study includes the comparatively small sample size which limited us from carrying out further subtypes-specific studies. The study strengths include the hospital-based study plan and the enrollment of pregnant women, which characterized sexually energetic females within ages between 18 and 43 years. This is comparable to women used in related studies presenting HPV seroprevalence peak in ages <30 year [9, 57]. Cohorts of pregnant women have been employed to account for STIs in sexually energetic population [39]. This perhaps characterize females with usual dissemination of cytological results [58]. This present study and other previous studies have focused on the natural history of serum IgG responses to HPV types 6, 11, 16 and 18 [59-61]. IgA being the second most abundant isotype in serum, plays an important role for protection against pathologic agents at mucosal sites [61, 62]. A lot of cross-sectional studies have established that IgA reactions specific for HPV VLPs correlate with IgG reactions or with the detection of HPV DNA of that type [61-67]. Thus, the period between the acquisition of HPV infections and the development of specific IgA antibodies and the duration of these responses remains largely undefined [61]. Longitudinal studies in the future are necessary to characterize the natural history of immune responses [61].
5. Conclusions
- This study has further shown high seronegativity outcomes to specific-IgG antibodies against HPV among pregnant women. The 100.0% seronegativity among the pregnant women indicates a 100.0% vulnerability to infections with the four HPV genotypes. It can be inferred that these pregnant women had no natural exposure to, at least, one of HPV-6/11/16/18 subtypes or had not seroconverted as at the time of sampling. The study obviously show that though the seropositivity rates were higher amongst pregnant women elsewhere in Nigeria, none of the pregnant women evaluated in this study had IgG-specific antibodies to the studied HPV VLPs (HPV subtypes 6/11/16/18). Our finding reveals vulnerability of a larger populations of pregnant women to HPV infections in Ibadan, Nigeria. Consequently, the non-vaccinated women are at higher risk of HPV infections as compared to vaccinated. This supports the need for routine and prompt screening of pregnant women for HPV infection and HPV-related manifestations in Nigeria. However, HPV re-vaccination is advocated. Epidemiological records of hrHPV infection will aid to evaluate vaccine program and efficacy.
ACKNOWLEDGEMENTS
- The authors sincerely acknowledge the support of Management and staff of Adeoyo Maternity Teaching Hospital, Yemetu, Ibadan, Nigeria; St. Mary Specialist Hospital, Eleta, Ibadan, Nigeria and University College Hospital, Ibadan, Nigeria. We also thank Dr. MO Adewumi for his unrelenting support and cooperation. We also thank Mr. NA Fashina and Miss Adesokan GH for the samples and all the pregnant women for their consent, cooperation and participation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML