-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Public Health Research
p-ISSN: 2167-7263 e-ISSN: 2167-7247
2015; 5(6): 171-176
doi:10.5923/j.phr.20150506.01

Relationship between Maternal Blood Lead, Cadmium, and Zinc Levels and Spontaneous Abortion in Sudanese Women
Khalid Mohamed Adam1, Sahar Abdalla Abdaltam2, Aisha Mustafa Noreldeen2, Wafaa Alfadil Alseed2
1Department of Molecular Biology, Faculty of Medical Lab Sciences, Alneelain University, Khartoum, Sudan
2Department of Clinical Chemistry, Faulty of Medical Lab Sciences, Alneelain University, Khartoum, Sudan
Correspondence to: Khalid Mohamed Adam, Department of Molecular Biology, Faculty of Medical Lab Sciences, Alneelain University, Khartoum, Sudan.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

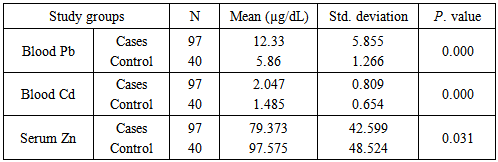
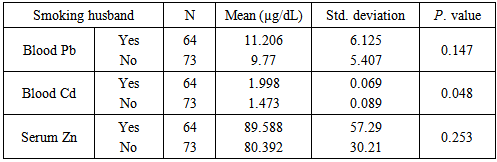
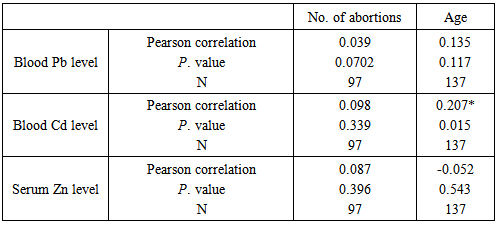
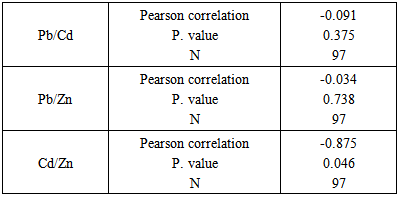
Background:Spontaneous abortion presents itself as a major reproductive health problem in the world as general and Sudan in particular, where the etiology behind this problem is seldom addressed. This study seeks to explore the relationship between maternal levels of Pb, Cd, and Zn, and the risk of spontaneous abortion in Sudanese women. Materials and methods:In a descriptive case control study the level of maternal blood Pb, Cd, and Zn was measured with Buck scientific 210VGP Atomic Absorption Spectrophotometer in blood specimens collected from 97 recently aborted women with gestational age at abortion ≤ 20 weeks, designated as cases, and 40 healthy pregnant women at gestational age >20weeks, designated as controls. Results:Recently aborted women showed high levels of Pb (12.33±5.855), Cd (2.047±0.809) when compared to control group with Pb (5.86±1.266) and Cd (1.485±0.654) with P. value 0.000. The cases also showed lower Zn level (79.373±42.559) when compared to the control group (97.575±48.524), P. value 0.031. Cadmium was the only element to statistically significant difference when women with smoking husbands (1.998±0.069) were compared to women with non-smoking husbands (1.473±0.089), P. value 0.048. On the other hand, when correlations were assessed, neither of the 3 elements showed significant correlation with number of abortions, while Cd was the only element to show correlation with the maternal age (r=0.207, P. value 0.015). Cadmium and Zinc were negatively correlated (r=-0.875, P. value 0.046), whereas, neither Pb and Cd, nor Pb and Zn showed statistically signification correlation. Conclusions: It can be concluded that high levels of Pb, Cd and low level of Zn increase the risk of spontaneous abortion in Sudanese women. The findings suggest passive smoking increases maternal Cd blood level. Considering the accumulative nature of Cd, it is concluded that maternal blood level of the element increases with age.
Keywords: Lead, Cadmium, Zinc, Spontaneous abortion, Sudanese
Cite this paper: Khalid Mohamed Adam, Sahar Abdalla Abdaltam, Aisha Mustafa Noreldeen, Wafaa Alfadil Alseed, Relationship between Maternal Blood Lead, Cadmium, and Zinc Levels and Spontaneous Abortion in Sudanese Women, Public Health Research, Vol. 5 No. 6, 2015, pp. 171-176. doi: 10.5923/j.phr.20150506.01.
Article Outline
1. Introduction
- The involuntary loss of a fetus weighing 500 gram or less before the 20th week of pregnancy is called spontaneous abortion or miscarriage according to the World Health Organization [1]. Although abortion is a morally, culturally and politically sensitive issue in many parts of the world, it remains a public health concern, with a rate of 14 unsafe abortions per 1000 women aged 15-44 years, and an estimated 21.6 million unsafe abortions took place worldwide in 2008 [2, 3]. Despite the actual cause behind spontaneous abortion usually goes undetected, there are number of factors implicated in the etiology of spontaneous abortion, this include, genetic predisposition, infection, immunological factors, uterine anomalies, maternal age, poor maternal diet, and environmental factors [4-6]. Of all environmental factors, lead (Pb) and cadmium (Cd) were classified among the top 7 most hazardous heavy metals, with lead coming second and cadmium 7th [7, 8].Environmentally, lead and cadmium have been widely dispersed and their levels in air and soil are increasing due to anthropogenic activities, such as burning of fossil fuels, industrial wastes, use of chemical fertilizers and mining activities [9-11]. The major routes of human exposure to these heavy metals are polluted food, water, inhalation of lead in automobile emissions. In addition, tobacco smoking can be an important source of cadmium exposure [12]. The toxic effect of lead was shown to manifest mainly in the haematological system, the central nervous system, and the renal system [12]. Although the mechanism remains obscure, the role of elevated blood lead level in spontaneous abortion was also reported [5, 7, 13]. On the other hand, the effect of cadmium as a causative factor of spontaneous abortion was attributed mainly to its lipid peroxidation activity which results in intrauterine oxidative stress [14].Zinc (Zn) is an essential micronutrient for human health. The historical belief that zinc deficiency is rare because zinc is ubiquitous was invalidated after number of clinical trials reported widespread incidence of zinc deficiency mainly due to nutritional causes [15]. Numerous studies have investigated the effect of zinc supplementation and deficiency on pregnancy outcomes [16, 17, 7]. The aim of the present study was to explore the relationship between the maternal blood level of lead, cadmium, and zinc and spontaneous abortion in Sudanese women, this study included a relatively large number of cases and it is one of the first attempts to investigate the role of heavy metal in spontaneous abortion in Sudan.
2. Materials and Methods
- In the present descriptive case control study a convenience sampling technique was used to enroll 97 women underwent recent (less than week) medically unexplained spontaneous abortion with gestational age at abortion ≤ 20 weeks and an average age of (22-39) years, and history of one, two or more miscarriages, designated as cases, and 40 healthy pregnant women at gestational age >20 weeks with average maternal age (18 – 40) years, with no history of abortion designated as controls. Women suffer from chronic disease, under medication or micronutrient supplementations were excluded. Both cases and controls groups were recruited from the Obstetrics and Gynecology clinics of Omdurman, Ibrahim Malik, and Bashair Hospitals, between February 2014 and May 2015. Prior to the collection of demographic data by direct personal interview and clinical data from medical reports, an informed written consent from each subject and ethical approval from the ethical and technical committee of faculty of medical lab sciences, Alneelain University were obtained. For each participant 10 ml of venous blood sample were collected under aseptic conditions, divided into two parts, the first part was collected in heparin container for analysis of lead and cadmium, and the second part in plain container from which serum was obtained for zinc analysis. All chemicals and reagents used were analytical reagent grade, glassware were washed with deionized water, then soaked in 20% nitric acid overnight and then thoroughly rinsed with deionized water. Blood lead and cadmium were measured with Buck Scientific 210VGP Atomic Absorption Spectrophotometer, using protocols provided by the manufacturer.
2.1. Lead Measurement
- 100μL of blood sample were diluted with 400μL of diluent (0.25% Triton X-100, 2000 ppm ammonium dihydrogen phosphate, and 750 ppm magnesium nitrate). A 0, 0.01, 0.05, 0.1, 0.25, 0.5 mg/L lead standards were made with the diluents, and then 20μL loads were analyzed with the Graphite Furnace Atomic Absorption Spectrophotometer. The detection limit is 2.5μg/dL.
2.1.1. Instrument Parameters
- 283.3nm wavelength, 7A slit, D2 background correction, Peak Height, Argon purge (~50ml/min), Auto-Zero off, grooved furnace tube. Dry: Ambient to 125°C in 15 second ramp, 5 second hold. Ash: 125°C to 600°C in 45 second ramp, 20 second hold. Atomize: 600°C to 2400°C in fast ramp or step, 5 second hold.
2.2. Cadmium Measurement
- Dilutions of stock cadmium solution were prepared using Ammonium Phosphate solution (40%), the calibration standards contained 0.5% (v/v) nitric acid. Samples were prepared under direct aspiration method, and they contained 0.5% (v/v) nitric acid.
2.2.1. Instrument Parameters
- Drying Time and Temp: 30 sec-125°C. Ashing Time and Temp: 30 sec-500°C. Atomizing Time and Temp: 10 sec-1900°C. Purge Gas Atmosphere: Argon. Wavelength: 228.8 nm.The detection limit is 0.01μg/dL.
2.3. Zinc Measurement
- Zinc standards were prepared by diluting the stock standard solution in the (STD concertinas) for zinc, with 5%glycerol (v\v). Serum sample was diluted 1:5 with deionized water.
2.3.1. Instrument Parameters
- Drying Time and Temp: 30 sec - 125°C. Ashing Time and Temp: 30 sec - 400°C. Atomizing Time and Temp: 10 sec - 2500°C. Purge Gas Atmosphere: Argon. Wavelength: 213.9 nm.Detection limit is 0.005μg/dL.
2.4. Data Analysis
- Student’s T-test was used to compare level of heavy metals between the two arms of the study, and between women in the cases group according to the smoking habit of the husband. Associations were assessed with Pearson correlation test. The statistical significance was set at P<0.05. Data was analysed using SPSS.16.
3. Results
- Comparison of blood level of heavy metals between the two arms of the present study showed a statistically significant difference in the level of lead in cases (12.33±5.855) and controls (5.86±1.226) with P. value 0.000, and in the level of cadmium in cases (2.047±0.809) and controls (1.485±0.654) with P. value 0.000, whereas serum zinc showed a significantly lower levels in the cases (79.373±42.599) than that of the controls (97.575±48.524) with P. value 0.031, as shown in table 1, figure 1 & 2.
|
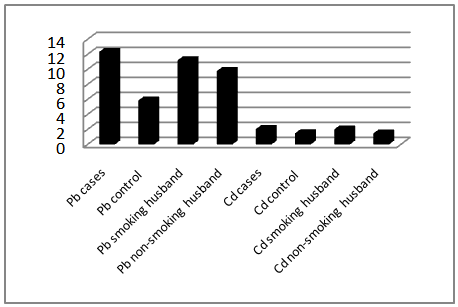 | Figure 1. Mean of blood Pb and Cd levels in study groups |
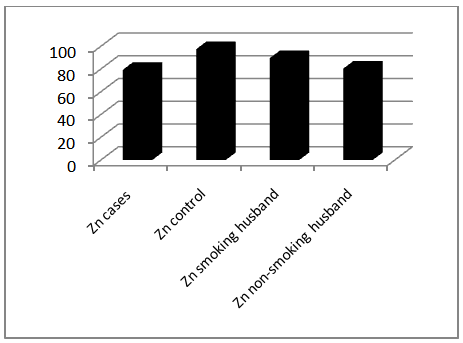 | Figure 2. Mean of serum Zn levels in study groups |
|
|
|
4. Discussion
- Lead is a bluish-gray heavy metal that occurs naturally in the Earth’s crust [18]. However, most of the high contaminating levels found throughout the environment come from human activities. From a public health prospective lead was placed among the top most hazardous substances in the world [18]. The toxic effect of lead on human health has been known for centuries [19]. Despite, the developing nervous system, the hematologic and cardiovascular system, and the kidneys being the most toxicity-prone organs [6, 20, 21], effect of the heavy metal on male and female reproductive systems and growing fetus is well documented [22-26, 5, 6, 19].The findings of the present study showed a significantly high blood lead level in aborted women when compared to their pregnant counterparts, this goes in accordance with several previously reported results [6, 7, 27, 28] with the presumption that high levels of maternal blood lead cross the placental barrier [7] and cause extremely adverse effects on the growing embryo that result in either abortion [29], intrauterine deaths, still-birth [30], preterm labor and growth retardation [31], or low birth weight babies [28]. The high level of blood lead showed by aborted women indicates a possible exposure to lead polluted source. The primitive agricultural methods followed by many farmers in Sudan and the excessive use of pesticides in farms located mostly next to the residential areas, along with the extremely old drinking water pipeline system and the use of lead containing paints, present possible sources of lead poisoning in the present study. Although cigarette smoking reported as a potential source of lead exposure [32], in the present study blood lead level showed no significant difference between women with smoking husbands and those with non-smoking husbands. Blood lead level in the aborted women showed no correlation with neither number of abortions, nor maternal age at abortion which disagrees with previously reported results [16], in the present study the absence of correlation between number of abortions and maternal age at abortion can be attributed to the fact that lead has a relatively short half-life in the blood; approximately 30 days [33] which means little chance of accumulation in case of exposure to low doses, another reason could be that 94% of the total body burden of lead in human adults is found in the bone [34], therefore, the accumulative nature of the lead -which means the increment of lead with age or number of abortions- cannot be driven from blood lead level alone. The second heavy metal investigated in the present study was cadmium, which is one of the most important environmental pollutant affects mostly renal, neurological, skeletal systems. The heavy metal is also implicated with other toxic effects, such as reproductive toxicity, genotoxicity, and carcinogenicity [35]. Cadmium is disseminated throughout the environment by human activities such as mining, fuel combustion, smoking, use of Cd based pigments, and stabilizers used in plastics. In agreement with the findings of several studies [5, 7, 14], the results in the present study showed a significantly high levels of blood cadmium in the aborted group which implies a possible role of cadmium in the abortion process, this role can be explained by number of postulated mechanisms through which Cd exerts its toxic effect on pregnant women, this include lipid peroxidation, the primary mechanism of Cd toxicity [14], Cd is also known to cause placental necrosis and hemorrhage [36, 37], cell membrane proteins and enzymes also get affected when Cd binds to SH group of these proteins, Cd also compete with Zn and other micronutrients for placental absorption causing deficiency of these micronutrients [38, 39]. The elevated levels of blood Cd in the aborted women indicative of possible heavy metal poisoning, can be attributed to the indifferent attitude towards the use of possibly cadmium-contaminated plastic bags for carrying hot foods and drinks in Sudan. The level of cadmium in the cases based on the smoking habit of the husband, showed higher levels in women with smoking husbands when compared to women with non-smoking husbands, this goes in concordance with previously reported results [7]. Both active and passive smoking have been reported as source of Cd poisoning [38], therefore, the elevated levels of heavy metal in this case can be attributed to passive smoking of pregnant women. The fact that erythrocytes are one of the major sites of Cd accumulation in the human body [35, 40, 14] along with the relatively long half-life of Cd, approximately 10 -30 years [7] explain the accumulative nature of blood cadmium, and hence, the positive correlation between blood Cd level and maternal age at abortion shown in the present study.Zinc on the other hand, is an essential micronutrient with important role in cellular protein synthesis, nucleic acid metabolism, and cell division [41], deficiency of maternal blood zinc has been implicated with spontaneous abortions, congenital malformations, and intrauterine growth retardation [17]. In the present study maternal serum zinc showed deficient levels in aborted women when compared with the control group, this goes in agreement with previously reported results [15-17] the reason behind low Zn levels in the cases group could be merely nutritional, or due to decreased Zn bioavailability and absorption as a result of the competing nature of cadmium, as the correlation result of the two elements showed a strong negative correlation. Serum Zn level based on the smoking habit of the husband showed lack of significant difference between the two groups. The micronutrient level also showed no correlation with number of abortions or maternal age, as Zn is a ubiquitous element, therefore, persistence of deficiency will probably not last for long. Lack of correlation was also observed between Pb/Cd, and Pb/Zn.
5. Conclusions
- Although the actual cause of spontaneous abortion in Sudanese women usually goes undetermined, it can be concluded that maternal levels of Pb, Cd, and Zn play major role in the outcome of the pregnancy. The results suggest that Sudanese pregnant women in the present study were exposed to polluting levels of Pb, and Cd. The results are suggestive of an accumulative nature of Cd with age, and a role of passive smoking in accumulation of this heavy metal, and thus, an indirect causative effect of spontaneous abortion. It is also safe to conclude that deficient levels of maternal serum Zn indicative to increase risk of spontaneous abortion, nonetheless further studies are needed to confirm these results.
AKNOWLEDGEMENTS
- We are highly indebted to staff of clinical chemistry department, faculty of medical lab science – Alneelain University, and the staff of Obstetrics and Gynecology clinics of Omdurman, Ibrahim Malik, and Bashair Hospitals.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML