-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Public Health Research
p-ISSN: 2167-7263 e-ISSN: 2167-7247
2015; 5(2): 58-65
doi:10.5923/j.phr.20150502.03
Prevalence of HIV among Pregnant Women in Rumubiakani, Port Harcourt, Nigeria
Okerentugba PO, Uchendu SC, Okonko IO
Medical Microbiology Unit, Department of Microbiology, University of Port Harcourt, Port Harcourt, Nigeria
Correspondence to: Okonko IO, Medical Microbiology Unit, Department of Microbiology, University of Port Harcourt, Port Harcourt, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
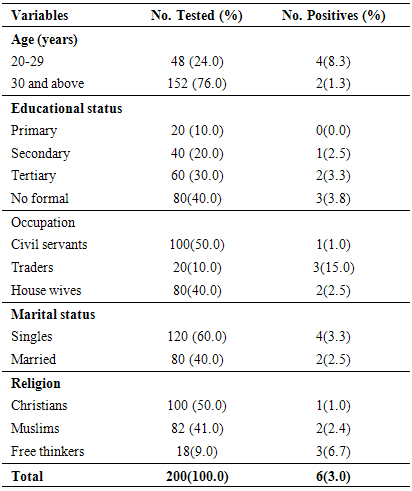
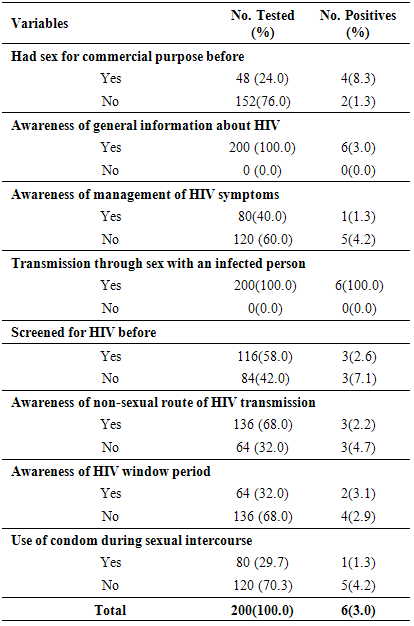
This study is aimed at determining the prevalence of HIV among pregnant women in Rumubiakani, Port Harcourt, Rivers State, Nigeria. The purpose of the study was explained to the pregnant women attending Antenatal clinic (ANC) in Obio Cottage Hospital, Rumubiakani. Those who consented to the study were given questionnaire. Blood samples were also collected from 200 pregnant women and screened for antibodies to HIV using Determine HIV-1/2 and Stat Pak HIV-1/2 rapid strips. The overall prevalence of HIV was found to be 3.0% (n=6). Of which 4(66.7%) were due to infection by HIV -1 while 2(33.3%) were due to infection by HIV-2. Higher prevalence of 8.3% was found among age-groups 20-29 years compared to 1.3% recorded among >30 years. Participant who had never had any form of formal education had the highest prevalence of HIV [3(4.0%)] while those who had only primary level of education had zero prevalence [0(0.0%)]. Those who attested to having gone through secondary and tertiary levels of education had 1(2.5%) and 2(3.3%) prevalence respectively. Higher prevalence was found among adults 3(4.0%), illiterates 3(4.0%), traders 3(15.0%), singles 4(3.3%) and free thinkers 3(10.7%). HIV seropositivity significantly associated with behavioral variables such as had sex for commercial purpose before (8.3%), no HIV screening (7.1%), no awareness of non-sexual route of HIV transmission (4.7%), no condom use (4.2%) and no awareness of management of HIV symptoms (4.2%). Also 200(100.0%) of the participants acknowledged being aware of the existence of HIV. All (100.0%) agreed that to have sex with an infected partner could transmit the virus. However, 58.0% acknowledged being tested for HIV before. Sixty-eight percent (68.0%) of the participants were aware of other routes of transmission of the virus aside sex; they admitted sharing sharp unsterilized objects such as razor blades could be a risk factor. Forty percent (40.0%) of participants admitted they knew how to manage symptoms of the infection and 32.0% were aware of HIV window period. This study has further confirmed the presence of HIV among pregnant women in Port Harcourt, Rivers State, Nigeria.
Keywords: HIV, Antibodies, HIV/AIDS awareness, Pregnant women
Cite this paper: Okerentugba PO, Uchendu SC, Okonko IO, Prevalence of HIV among Pregnant Women in Rumubiakani, Port Harcourt, Nigeria, Public Health Research, Vol. 5 No. 2, 2015, pp. 58-65. doi: 10.5923/j.phr.20150502.03.
1. Introduction
- As HIV/AIDS is a major public health problem in Nigeria, the pandemic is dominated by HIV-1, which was discovered in 1983 [1]. In 1987, HIV-2 was discovered which is very common in West Africa and has not shown any significant spread from there [1]. Nigeria has the largest population in Africa with a population of over 150 million and HIV prevalence of 4.6% in 2008 [2-3]. It is estimated that 2.95 million individuals live with HIV/AIDS in Nigeria [4] and integrated control efforts are immeasurably needed [3, 5-6].A more serious challenge today, is the growing infection rates among the adolescents in sub-Saharan Africa [7]. There is great concern about the spread of HIV epidemic in or within the adolescent population [8-9]. According to Unuigbe et al. [10], Fawole et al. [11], Musa et al. [12] and Srivastava and Srivastava [13], most youths become sexually active before marriage, many while still in their teens had begun sexual activity.HIV in pregnant women is an important public health concern. Antenatal screening for HIV should routinely be offered to all pregnant women, as early diagnosis and management is important both to prevent transmission to the child and the mother’s health. Thus, this study aimed at determining the prevalence of HIV among pregnant women in Obio Cottage Hospital, Rumubiakani, Port Harcourt, Rivers State, Nigeria.
2. Methods
- Study AreaThis study was conducted at the Obio Cottage Hospital, Rumuobiakani, Port Harcourt, Rivers State, Nigeria. This was a cross-sectional, consecutive health-facility-based study. In order to obtain a study sample representative of Rumuobiakani area of the state, pregnant women attending the attending Obio Cottage hospital in Rumuobioakani, Port Harcourt, Nigeria were selected. To further buttress this, it is one of the mostly utilized hospitals in Rivers State, Nigeria.Ethical considerationsEthical approval was also given by the management of Obio Cottage hospital. Designated hospital staff members clearly explained, in English or in the individual’s dialect, the objectives and procedures of the study to each prospective participant. Written or oral informed consent was taken from the subjects before enrolment into the study. The participants were assigned identification numbers and were assured that all information obtained would be treated with the utmost confidentiality and used solely for the purpose of this research. Patient Eligibility and Inclusion criteriaPregnant women who consented to participate were consecutively recruited for the study. Pregnant women attending the antenatal clinic present at the participating hospital who voluntarily provided informed consent/assent to participate in the study were eligible. All those unwilling to provide informed consent/assent for participating in the study were not eligible. The study was carried out according to ethical research standards. Study PopulationA total of two hundred consented pregnant women were enrolled in this study. Of which, 152 were in age groups 30-35 years while 48 were in age groups 20-29 years of age. Informed consent was obtained from each patients and relevant confidentiality was maintained throughout the study. Sample size determinationThe formula n=Z2PQ/d2 was used to derive the desired sample size. Where n is the desired sample size, P is the expected prevalence in the target population, Q is 1-P, Z is 1.96; standard error, d is the level of statistical significance (0.05). A P-value of 7.3% was used representing maximum uncertainty for Rivers State during the last National HIV sentinel study in 2010 [14, 15]. Hence, the estimated sample size was 104 with an additional 10.0% sampled to take care of data inconsistencies [16], providing a total sample size of 115 which was approximated to 200. Thus, N= 200 participants were recruited for this study. Sample CollectionQuestionnaires were given to the studied population who volunteer for the study. Confidentiality as assured the targeted groups was maintained. The questionnaires administered included their demographic profile (age, religion, marital status, and educational level), sexual history (marital status, condom use and their HIV awareness level), awareness of existence of HIV, knowledge about management of HIV infection and non-sexual route of HIV transmission). This information was obtained using coded questionnaires. The initial step was to explain in details the entire procedure of the study. All consented pregnant women in Obio Cottage Hospital that volunteered were then administered the coded questionnaires. This was followed by blood collection using sterile syringes which was later transferred into anticoagulant bottles. The bottles were arranged in a collection bode and sent to the medical laboratory for HIV antibody screening. Privacy and confidential were observed as wells as strict aseptic measure. Two hundred blood samples were collected for this study. Venous blood was obtained into non-anticoagulated tubes. The samples were centrifuged at 2000 resolution per minutes (rpm) for 5 minutes to obtain sera. The sera were stored at -20℃ for serologic assay of HIV. Serologic AssayDetermine HIV-1 and HIV-2 screening kit was used in this study. This is an immunochromatography (rapid) method for quantitative detection of antibodies of all isotopes (IgG, IgM, IgA) specific to HIV-1 and HIV-2 simultaneously in serum. The test was carried out according to the manufacturer’s specifications. Data analysisThe prevalence for HIV-1 and HIV-2 antibodies was calculated by using pregnant women with positive samples as numerator and the total numbers of pregnant women enrolled in this study were the denominator. The generated data were presented in descriptive statistics. The data generated were further subjected to Fisher’s Exact Test for comparison of proportions to determine any significant relationship between infection rate and demographical characteristics of the subjects.
3. Results
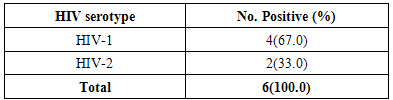
- Overall prevalence During the study, a total of two hundred (200) samples were screened for antibodies of HIV. Six (3.0%) samples were positive for HIV, of which 4(66.7%) were due to infection by HIV-1 while two (33.3%) were due to infection by HIV-2 (Table 1).
|
|
|
4. Discussion
- The epidemic of AIDS causes by infection with HIV has remained a major public health problem globally, wreaking devastation on millions of families and communities. The HIV/AIDS pandemic is now recognized as unprecedented challenge to global health and has generated an extraordinary mobilization for the prevalence and central of pandemic [4]. This study was carried out to determine the seroprevalence and the possible risk factors of HIV among 200 pregnant women attending ANC in Obio Cottage Hospital Rumubiakani, Port Harcourt, Rivers State Nigeria.The overall prevalence of HIV in this study was 3.0% (n=6). This prevalence rate is lower compared to that of National prevalence of 3.6% and 7.3% in Rivers State. Four (67.0%) of them was due to infection by HIV-1 while 2(33.0%) was due to infection by HIV-2. Other studies in West Africa show 0.4% HIV-1 and 0.2% HIV-2 in northern Benin [1, 17], 6.6% HIV-1 and 0.9% HIV-2 in central Benin [1], 25% HIV-2 and 5% HIV-1/HIV-2 in Mauritania [1, 18], 65%HIV-1, 24% HIV-2 and 11% HIV-1/HIV-2 in Senegal [1, 19] and 12.1% HIV-1, 0.5%HIV-2 and 1.6% co-infection in north western Nigeria [1, 20].The low prevalence (3.0%) observed in this study may have been accounted for by the fact that the sampling was done in one hospital only. Different studies to determine the prevalence of HIV among different populations have been carried out. A prevalence of 47.54% was reported for HIV in Benin City, Nigeria [21]. Pennap et al. [22] reported 38.65% prevalence rate of HIV and AIDS in Keffi and environs, Nassarawa State, Nigeria. Motayo et al. [23] reported 13.6% among patients and 28.6% among pregnant women in Ibadan. A prevalence of 12.0% was also reported among patients with pyrexia of unknown origin and 10.8% among STD patients in Ibadan, Nigeria [23]. A zero seroprevalence rate was reported for HIV among blood donors in Ibadan [24] and 11.0% was reported among pregnant women in Port Harcourt, Nigeria [25]. HIV/AIDS is a pandemic affecting the rich and poor, educated and the illiterate, the married and the single. The 3.0% prevalence reported in this study is lower than the 4.1% HIV prevalence reported previously among traders in Port Harcourt [26]. It is lower than the prevalence of 5.1% reported in Cameroon in 2010 [1] and the 5.8% reported in Maiduguri, Nigeria [27]. This finding also differs from the 6.0% rate reported in Jos, Nigeria [28] and the 4.55% reported in Cameroon [29]. It is also lower than the 47.54% reported in Benin City, Nigeria [24]. It is far lower than the 38.65% reported in in Keffi and environs, Nassarawa State, Nigeria [25] and the 13.6%, 28.6%, 12.0% and 10.8% reported in Ibadan, Nigeria among patients, pregnant women, patients with pyrexia of unknown origin and STD patients respectively [23].The 3.0% reported in this study is comparable to the 3.1% reported in Osogbo, Nigeria [30]. This rate also differs from the 3.5% seroprevalence reported in Enugu [31]; the 3.8% seroprevalence reported in Dar es Salaam [32]. It is higher than the 0.0% seroprevalence reported for HIV in Ibadan [24].The study showed age-related differences (p<0.05). In this study, HIV prevalence was higher among pregnant women within age groups 20-29 years (8.3%) than age group 30 years and above (1.3%). Results from previous studies have shown that age has always proved to be the most important factor in all epidemiological studies [33-37]. The difference in prevalence of HIV in various age groups indicates that this factor play an important role in the prevalence rate. Our study confirmed that majority of those who contract HIV fell under the age below 30 years [33, 37]. Akinjogunla and Adegoke [34] reported a significant difference in the age of the individuals with HIV. Laah and Ayiwulu [35] who reported higher seroprevalence rate of HIV in age group 20-34 years. Macpherson et al. [38] reported in a higher prevalence of HIV among children greater than 15 years of age in Canada. The study by Middelkoop et al. [36] showed a high force of infection among adolescents, positively associated with increasing age. However, this present study deviated from that of Okonko et al. [39] who reported no significantly association with HIV- 1/2 seropositivity and age. This was also not in line with the findings of Alikor and Erhabor [40] and Sule et al. [41], who reported no statistically difference in age.The study showed occupation-related differences (p<0.05). HIV prevalence was higher among traders (15.0%) than house wives (2.5%) and civil servants (1.0%). Based on occupation, three categories of participants were studied; civil servants, traders and house wife, results obtained showed that traders had the highest prevalence compared to civil servants with the lowest prevalence. Previous studies found that women with low income and low socioeconomic status are more likely to access antenatal care late or be unbooked [25, 42-43].According to Kagimu et al. [44], it is possible that religious practices such as circumcision could partly explain the differences. However, it is not clear whether those who are more religious and adhere to their religious practices have a lower HIV prevalence rate compared to those who do not [44]. The present study showed religion-related differences (p<0.05). HIV prevalence was higher among free thinkers (6.7%) than Muslims (2.4%) and Christian (1.0%). This high prevalence observed in free thinkers could be attributed to the fact that their tradition allowed them to be polygamous in nature. This factor may predispose the free thinkers to a higher risk of becoming infected with the virus especially when marriage is frequently contracted without HIV screening. Christians on the other hand are taught abstinence and their belief ensures a man to a wife which makes them less prone sexually transmitted disease including HIV. This could be the possible explanation for the lower prevalence observed. It has been suggested by scholars that studies linking religiosity to serological markers of HIV infection are likely to increase understanding of the role of religion in HIV prevention [44, 45]. The finding of this study disagrees with Frank-Peterside et al. [46] who reported no religion differences in HIV prevalence. The 1.0% value reported for Christians in this study is far lower than the 3.6% reported by Kagimu et al. [44] in their study and the 3.4% reported earlier in their 2005 national sero-behavioral survey in Uganda [44, 47]. It is also lower than the 10.5% reported by Frank-Peterside et al. [43]. The 2.4% reported for Muslims in this study is lower than the 12.5% reported by Frank-Peterside et al. [43]. This is also lower than the 5.0% reported among Muslims in previous studies [44, 47]. There was no significant difference (p>0.05) between HIV seropositivity and educational status. According to the level of education, the highest prevalence of HIV was recorded for participants that were illiterate. This was closely followed by the less educated (secondary). This disagrees with Frank-Peterside et al. [25] who reported significant difference between educational status and HIV seropositivity. A study by Buseri et al. [26] found a correlation in terms of occupation and HIV seropositivity. Previous studies found that women with a low level of education were more likely to access antenatal care late or be unbooked.There was no significant difference (p>0.05) between HIV seropositivity and marital status. Results obtained shows that the prevalence of HIV to be 3.3% among singles and 2.5% among the married. This disagrees with the findings of Frank-Peterside et al. [43] who reported marital status differences in HIV prevalence of traders in Port Harcourt, Nigeria. The possible explanation for this trend in prevalence might be due to other contributing factor such as multiple sex partner, pre-marital and extra-marital sexual contacts which were common in Port Harcourt [43, 46, 48]. This finding is similar to that of Mbakwem-Aniebo et al. [48] who found HIV prevalence to be highest among singles. However, the finding of this study agrees with Frank-Peterside et al. [46] who reported no marital status differences in HIV prevalence in Port Harcourt, Nigeria and disagrees with Okonko et al. [49] who reported marital status associated HIV positivity in their study in Ibadan, Nigeria.HIV seropositivity significantly associated with behavioral variables such as had sex for commercial purpose before (8.3%), no HIV screening (7.1%), no awareness of non-sexual route of HIV transmission (4.7%), no condom use (4.2%) and no awareness of management of HIV symptoms (4.2%). Previous studies have also documented that youths indulge in many of the behaviors that promote HIV transmission, including having sex with multiple partners [48], having unprotected sexual intercourse, and using drugs or alcohol during sex [48, 50-54].One hundred percent of the participants were aware of HIV. This is in agreement with previous report by Mbakwem-Aniebo et al. [48] among fresh university students. It also corroborates previous findings by Orubuloye et al. [55] and Olowosegun et al. [56]. Olowosegun et al. [56] reported that 98.4% of the respondents in their study knew about HIV/AIDS. Participants also agreed that to have sex with an infected partner could transmit the virus. This finding supports that of Isibor and Ajuwon [57] on journalists' knowledge of AIDS. While in that of Olowosegun et al. [56], the perception of the respondents on HIV/AIDS is high, 90.4% believed that it is a serious deadly disease but lack the information that could help them to live dignified life.Madani et al. [58] reported that at diagnosis, some infected persons do not have prior knowledge of their HIV status. In this study, 58.0% acknowledged being tested for HIV before. Sixty-eight percent (68.0%) of the participants were aware of other routes of transmission of the virus aside sex; they admitted sharing sharp unsterilized objects such as razor blades could be a risk factor. Forty percent (40.0%) of participants admitted they knew how to manage symptoms of the infection and 32.0% were aware of HIV window period. This trend is similar to the reports by Yahaya [59], Olowosegun et al. [56] and Mbakwem-Aniebo et al. [48].Most youths engaged in sex without proper protection and awareness about sexually transmitted infections [13]. Risk factors associated with HIV, such as sex with a number of partners, clearly exist among adolescents and young adults, including those on university campuses [48, 60-61]. This study showed 4.2% prevalence of HIV among participants with low condom use. However, reports have shown that there are missed reactions to condom use. Some say it takes the pleasure from sex while some admitted to having irritation from using it. Others still feel it is better to protect one from being infected with HIV. Based on the results obtained from the study, it was observed that awareness about HIV among participants was limited to the sexual route of HIV transmission. High proportions 79% of participants were not aware that HIV infection could also be transmitted through blood transfusion. Youths with a reasonable knowledge of HIV may not perceive themselves to be at risk and may continue to engage in high-risk behavior [48, 62-63].
5. Conclusions
- This study however, further confirmed the presence of HIV-1 and HIV-2 antibodies among pregnant women in Port Harcourt, Nigeria. This calls for urgent and concerted efforts aimed at promoting behavioural, cultural and social changes that will reverse the current trend in the prevalence of HIV among the pregnant women.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML