Amawulu Ebenezer 1, 2, M. Aline Edith Noutcha 1, Samuel N. Okiwelu 1
1Department of Animal & Environmental Biology, University of Port-Harcourt, Port Harcourt, Nigeria
2Department of Biology Isaac Jasper Boro College of Education, Sagbama, Nigeria
Correspondence to: Samuel N. Okiwelu , Department of Animal & Environmental Biology, University of Port-Harcourt, Port Harcourt, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Abstract
Background: Entomologic Inoculation Rate (EIR), the product of vector biting rate and the sporozoites rate or the proportion of mosquitoes with sporozoites in the salivary glands has been strongly correlated with malaria prevalence. This study was undertaken to determine whether mosquito collection method significantly affected EIR estimates. Method: Study was conducted in 7 local Government Areas (LGAs) of Bayelsa State, across 3 eco-vegetational zones (fresh water forest, brackish water swamp forest, mangrove coastal water forest). Three methods were used for mosquito collections: Pyrethrum Spray Catch (SPC), Human Bait Catch (HBC) (Indoor), Human Bait Catch (Outdoor). EIR was calculated as per standard method. Results: EIR means obtained by PSC and HBC (Outdoor) methods were not significantly different (F=4.989; df=2; p<0.05), but significant differences were obtained between PSC and HBC (Indoor) estimates. These differences were also significantly influenced by eco-vegetational zones (F=3.92; df=2’ p<0.05). Conclusions: It is advisable to compare EIRs obtained by the same mosquito collection methods as indices of malaria prevalence.
Keywords:
Vector Collection Methods, Entomological Inoculation Rates (EIRs), Malaria, Indoor/Outdoor, Ecovegetational zones
Cite this paper: Amawulu Ebenezer , M. Aline Edith Noutcha , Samuel N. Okiwelu , Does Variation in Malaria Vector Collection Methods Significantly Affect Entomologic Inoculation Rates?, Public Health Research, Vol. 5 No. 1, 2015, pp. 24-27. doi: 10.5923/j.phr.20150501.04.
1. Introduction
Malaria transmission intensity is often assessed by the entomologic inoculation rate (EIR), the product of the vector biting rate and the sporozoites rate (sr); which is the proportion of mosquitoes with sporozoites in the salivary glands [1]. EIR estimates the number of infective bites a person receives per unit time and thus the level of exposure of an individual to malaria parasites studies have shown strong correlation between EIR and malaria prevalence [2-4]; May et al. [5] reviewed EIRs across Africa. Unlike most other parts of the world where it is difficult to determine EIRs because of exceedingly low sporozoite rates, many valuable studies of transmission have been conducted in Africa, where sporozoite generally range from 1% to 20% [6]. The EIRs in ebdemic areas of Africa range from <1>1000 infective bites per year [7].Establishing the relationship between transmission intensity and health outcomes is crucial for the planning of long-term malaria control programmes. Unfortunately, this is fraught with methodologic difficulties. Smith et al. [8] noted that one of the important considerations is that the incidence of infection for Plasmodium falciparum malaria is much lower than entomologic inoculation rates (EIRs), especially at higher transmission levels. Moreover, biting rates of malaria vectors per host depend on his or her biomass and thus age. Smith et al. [8] proposed an algorithm for estimating human infection rates from the EIR with allowance for these two factors.To compare EIR and Malaria prevalence data collected simultaneously at the same sites; Beier et al. [4] undertook literature searches to identify relevant studies that met a priori a set of inclusion criteria. Studies conducted over at least one year; frequency of mosquito sampling at least monthly throughout the year or during periods of transmission for sites with seasonal patterns of transmission; standard methods such as human-biting catches, pyrethrum sparay catches, or Center for Disease Control light traps used for estimating biting rates; dissection or ELISA methods used for determining proportion of sporozoite-infected mosquitoes; and studies were conducted during periods when no mosquito control operations were in effect. Studies on the estimation of EIRs across the continent have involved different mosquito collection methods. These have been either singly: Bigoga et al. [9, 10] by the Human Landing Collection (HLC) indoors and outdoors in Cameroon and Amek et al. [11] by the Light Trap Collection (LTC) in Kenya; jointly : Overgaard et al. [12] by HLC and LTC in Equatorial Guinea and Wanji et al. [13] by HLC and Pyrethrum Spray Collection (PSC) in Cameroon. The thrust of most of these studies was on the comparison of seasonal EIRs and variation with location, except Overgaard et al. [12] who observed that EIRs were higher outdoors and mosquitoes obtained by HLC yielded higher EIRs than those by LTC. Studies were undertaken over a 12-month period to compare Entomologic Inoculation Rate data obtained using HBC and PSC methods simultaneously at the same location.
2. Methods
2.1. Study Area
The study was conducted in 7 Local Government Areas (LGAs), Bayelsa State, Nigeria. The vegetation comprises three eco-vegetational zones: freshwater swamp forest, brackish water swamp forest and mangrove coastal water forest. The topography of study area is characterized by a maze of creeks and swamps criss-crossing the low-lying plain. The study LGAs were Yenagoa, Sagbama and Kolokuma-Opokuma in the freshwater swamp forest; Ogbia, southern Ijaw, Ekeremor in the brackish water swamp forest and Nembe in the Mangrove coastal water forest. All LGAs were rural, with the exception of semi-urban Yenagoa, the State capital.
2.2. Mosquito collections
Details on PSC collections have been extensively described [14, 15]. Simultaneously, human bait catches were made by consenting trained individuals in each town/village. Mosquitoes were caught indoors and outdoors by two individuals at each location. Mosquitoes that landed to bite were spotted and caught immediately with a mouth aspirator. Collections were made on two consecutive nights in each quarter over 4 quarters (12 months). PSC and HBC collections were undertaken in the same compound.
3. Results
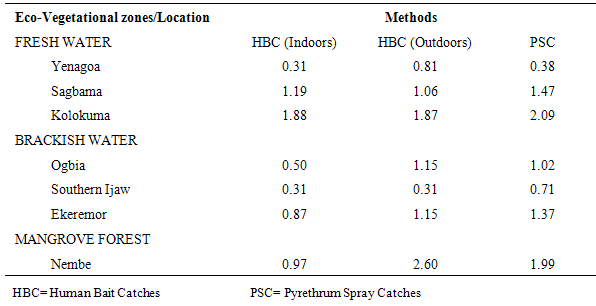
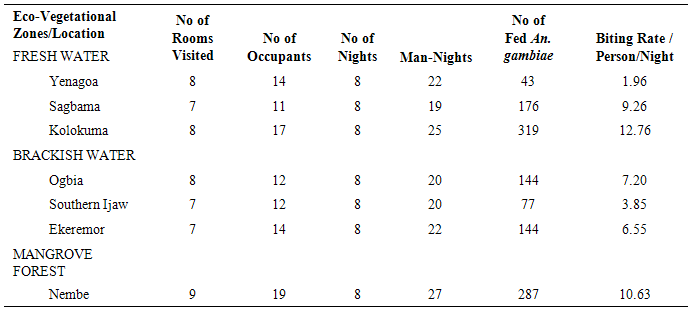
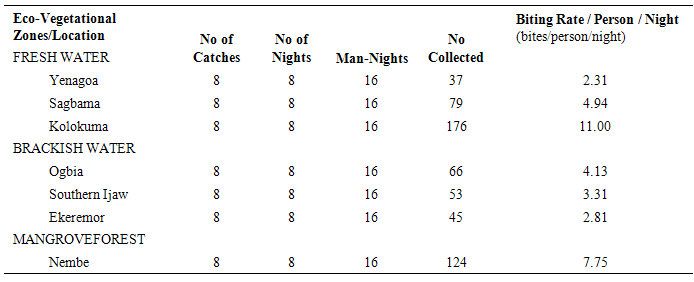
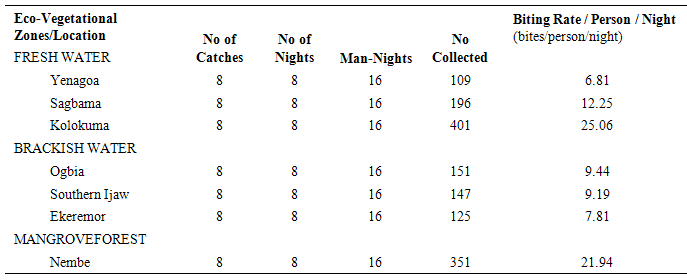
The results of the human biting, sporozoites and Entomologic Inoculation rates obtained by the three methods (PSC, HBC-Indoors, HBC-Outdoors) appear in Tables 1-7. EIR means obtained by PSC and HBC (Outdoor) methods were not significantly different; however, both were significantly different from EIR obtained by the HBC (Indoor) method (F=4.99; df=2; p<0.05). These differences were significantly affected by eco-vegetational zones (F=3.92; df=2; p<0.05) and location (F=9.24; df=4; p<0.05). Tests for significant differences in EIRs among collection methods and locations showed that there were significant differences; similar results were obtained among collection methods and zones (p<0.05).Table 1. Entomologic Inoculation Rates of Anopheles gambiae s.l. collected by different methods
 |
| |
|
Table 2. Man-Biting Rates of PSC-Collected Anopheles gambiae s.l. across Locations
 |
| |
|
Table 3. Man-Biting Rates of HBC-Collected (Indoors) Anopheles gambiae s.l. across Locations
 |
| |
|
Table 4. Man-Biting Rates of HBC-Collected (Outdoors) Anopheles gambiae s.l. across Locations
 |
| |
|
Table 5. Sporozoite Rates of PSC- Collected Anopheles gambiae s.l. across Locations
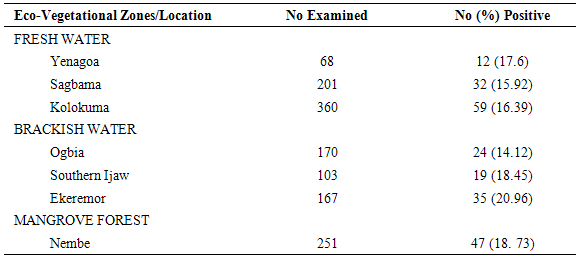 |
| |
|
Table 6. Sporozoite Rates of HBC-Collected (Indoors) Anopheles gambiae s.l. across locations
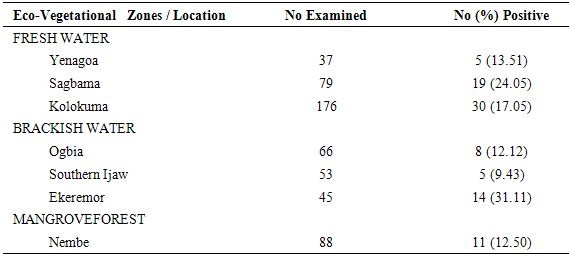 |
| |
|
Table 7. Man-Biting Rates of HBC-Collected (Outdoors) Anopheles gambiae s.l. across Locations
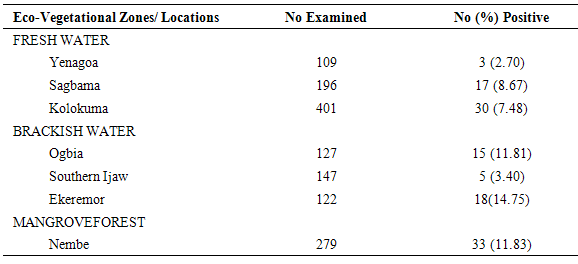 |
| |
|
4. Discussion
The higher HBRs and EIRs recorded outdoors from HBC in this study were also observed by Overgaard et al. [12] in Equatorial Guinea. The significant impact of location on EIR obtained in this study was also recorded by Bigoga et al. [10] in Cameroon. The significant differences in EIR means between HLC (outdoors)/ PSC and HLC (Indoors) indicate that collection method probably has an impact on EIR values. Overgaard et al. [12] also observed significant differences between EIRS obtained by HLC and LTC. Longterm studies are planned. It is advisable to compare EIRs obtained by the same collection methods as indices of malaria prevalence.
References
| [1] | Molineux L., Muir D.A., Spencer H.C., Wernsdorfer W.H. 1988. The epidemiology of malaria and its measurement. Malaria: Principles and Practice of Malariology. Wernsdorfer W.H., McGregor I Edingburgh. |
| [2] | Beier J.C., Oster C. N., Onyango F.K., Bales J.D., Chumo D. K., Koech D.V., Whitmire R.E., Roberts C.R., Diggs C.L., Hoffman S.L. 1994. Plasmodium falciparum Incidence Relative to Entomologic Inoculation Rates at a site proposed for testing Malaria vaccines in western Kenya. Am. J. Trop. Med. Hyg. 50:529-536. |
| [3] | Charlwood D., Smith T., Lyimo E., Kitua A., Masanja H., Booth M., Alonso P., Tanner M. 1998. Incidence of Plasmodium infection in infants in relation to exposure to sporozoites- infected anophelines. Am. J. Trop. Med, Hyg. 59: 243-251. |
| [4] | Beier J.C., Killeen G.F., Githure J.I. 1999. Short Report: Entomologic Inoculation Rates and Plasmodium falciparum malaria prevalence in Africa. Am. J. Trop. Med. Hyg. 61:9-13. |
| [5] | May S.L., Rogers D.J., Toomer J. F., Snow R.W. 2000. Annual Plasmodium Entomological Inoculation Rates (EIRs) across Africa: literature survey, Internet access and review. Trans. R. Soc. Trop. Med. Hyg. 94:113-127. |
| [6] | Wirtz R. A., Burkot T.R. 1991. Detection of malaria parasites in mosquitoes. Advances in Disease Vector Research. New York, Springer-Verlag. |
| [7] | Trape J.F., Roger C. 1996. Combatting malaria morbidity and mortality by reducing transmission. Parasitology Today 12:236-240. |
| [8] | Smith T., Killeen G., Lengeler C. & M. Tanner. 2004. Relationship between the outcome of Plasmodium falciparum infection and the intensity of transmission in Africa. Am. J. Trop Med Hyg 71 (Suppl 2): 80-86. |
| [9] | Bigoga J.D., Manga L. Titanji V.P.K, Coetzee M. & R.G. F. Leke 2007. Malaria vectors and transmission dynamics in coastal south-western Cameroon. Malaria Journal. 6:5-17. |
| [10] | Bigoga J.D., Nanfack F.M., Awono-Ambene P.H., Patchoke S., Atangana J., Otia V.S., Fondjo E., Moyou R.S. and R.G.F. Leke. 2012. Seasonal prevalence of malaria vectors and entomological inoculation rates in rubber cultivated area of Niete, South region of Cameroon. Parasites & Vectors 5:197-204. |
| [11] | Amek N., Bayoh N., Hamel M., Lindblade K., Giming J. E., Odhiambo F., Laserson K.F., Slutsker L., Smith T. & Vounatsou P. 2012. Spatial and Temporal dynamics of malaria transmission in rural western Kenya. Parasites & Vectors. 5:86-99. |
| [12] | Overgaard H.J., Reddy V.P., Abaga S., matias A., Reddy M.R., Kulkami V., Schwabe G., Segura L., Kleinschmidt I. & M.A. Slotman 2012. Malaria transmission sfter five years of vector control on Bioko Island, EquatorialGuinea. Parasites and Vectors 5:253-267. |
| [13] | Wanji S., Tanke T., Atanga S.N., Ajojina C., Nicholas T. & D. Fontenille. 2003, Anopheles species of mount Cameroon region: biting habits feeding behaviour and entomological inoculation rates. Trop. Med. Int. Health 8: 643-649. |
| [14] | Ebenezer A., S.N. Okiwelu, P.I. Agi, M.A.E Noutcha, T.S. Awolola & A.O. Oduola. 2012. Species composition of the Anopheles gambiae complex across eco-vegetational zones in Bayelsa State, Niger Delta region, Nigeria. J Vector Borne Dis 49: 164–167. |
| [15] | Ebenezer A., A. E. M. Noutcha, P. I. Agi, S. N. Okiwelu and T. Commander. 2014. Spatial distribution of the sibling species of Anopheles gambiae sensu lato (Diptera: Culicidae) and malaria prevalence in Bayelsa State, Nigeria. Parasites & Vectors, 7:32 http://www.parasitesandvectors.com/content/7/1/32. |

 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML





