| [1] | ASEAN Cosmetic Directive (ACD), 2008, ASEAN definition of cosmetics and illustrative list by category of cosmetic products. |
| [2] | Amasa, W., Santiago, D., Mekonen, S., Ambelu, A., 2012, Are cosmetics used in developing countries safe? Use and dermal irritation of body care products in Jimma Town, Southwestern Ethiopia. Journal of Toxicology, 1 - 8. |
| [3] | Lopaciuk, A., Loboda, M., 2013, Global beauty industry trends in the 21st century. International Conference, Zadar, Croatia. |
| [4] | Yeomans, M., 2012 Global beauty market to reach $265 bn in 2017 due to an increase in GDP, CosmeticDesign.com, http://www.cosmeticsdesign.com/Market-Trends/Global-beauty-market-to-reach-265-billion-in-2017-due-to-an-increase-in-GDP (accessed on 15 February 2014). |
| [5] | Draelos, Z.D., 2012, Are cosmetics safe?, Journal of Cosmetic Dermatology, 11: 249 - 250. |
| [6] | Hamilton, T., de Gannes, G.C., 2011, Allergic contact dermatitis to preservatives and fragrances in cosmetics. Skin Therapy Letter .com, http://www.skintherapyletter.com/2011/16.4/1.html (accesses on 5 May 2014). |
| [7] | Ayenimo, J.G., Yusuf, A.M., Adekunle, A.S., Makinde, O.W., 2010, Heavy metal exposure from personal care products, Bull Environ Contam Toxicol, 84; 8 - 14. |
| [8] | Read, S.I., 2012, Cosmetics and your health fact sheet, Office on Women's Health, U.S. Department of Health and Human Services, https://www.womenshealth.gov/publications/our-publications/fact-sheet/cosmetics-your-health.html (accesses on 27 April 2014). |
| [9] | Health Canada 2012, Guidance on heavy metal impurities in cosmetics, http://www.hc-sc.gc.ca/cps-spc/pubs/indust/heavy_metals-metaux_lourds/index-eng.php (accesses on 10 May 2014). |
| [10] | Roden, K., 2010, Preservatives in personal care products, Microbiology Australia. |
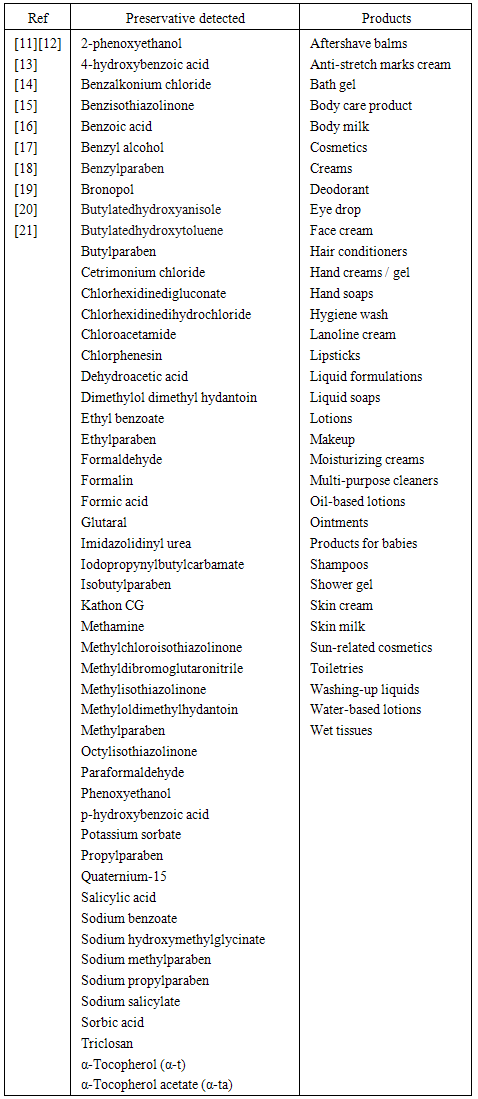
| [11] | Yazar, K., Johnsson, S., Lind, M.L., Boman, A., Lid ´en, C., 2010, Preservatives and fragrances in selected consumer - available cosmetics and detergents, Contact Dermatitis, 64: 265 - 272. |
| [12] | Zhang, Q., Lian, M., Liu, L., Cui, H., 2005, High-performance liquid chromatographic assay of parabens in wash-off cosmetic products and foods using chemiluminescence detection, Analytica Chimica Acta, 537: 31 - 39. |
| [13] | Sanchez-Prado, L., Alvarez-Rivera, G., Lamas, J.P., Lores, M., Garcia-Jares, C., Llompart, M., 2011, Analysis of multi-class preservatives in leave-on and rinse-off cosmetics by matrix solid-phase dispersion, Anal Bioanal Chem, 401: 3293 – 3304. |
| [14] | Rastogi, S.C., 2000, Analytical control of preservative labelling on skin creams, Contact Dermatitis, 43: 339 - 343. |
| [15] | Huang, H.Y., Lai, Y.C., Chiu, C.W., Yeh, J.M., 2003, Comparing micellarelectrokinetic chromatography and microemulsionelectrokinetic chromatography for the analysis of preservatives in pharmaceutical and cosmetic products, Journal of Chromatography A, 993: 153 - 164. |
| [16] | Memon, N., Bhanger, M.I., Khuhawer, M.Y., 2005, Determination of preservatives in cosmetics and food samples by micellar liquid chromatography, J. Sep. Sci, 28: 635 - 638. |
| [17] | Flyvholm, M.A., 2005, Preservatives in registered chemical products, Contact Dermatitis, 53: 27 - 32. |
| [18] | Lee, M.R., Lin, C.Y., Li, Z.G., Tsai, T.F., 2006, Simultaneous analysis of antioxidants and preservatives in cosmetics by supercritical fluid extraction combined with liquid chromatography–mass spectrometry, Journal of Chromatography A, 1120: 244 - 251. |
| [19] | Wu, T., Wang, C., Wang, X., Ma, Q., 2008, Simultaneous determination of 21 preservatives in cosmetics by ultra-performance liquid chromatography, International Journal of Cosmetic Science, 30: 367 - 372. |
| [20] | Xue, Y., Chen, N., Luo, C., Wang, X., Sun, C., 2013, Simultaneous determination of seven preservatives in cosmetics by dispersive liquid–liquid microextraction coupled with high performance capillary electrophoresis, Anal. Methods, 5: 2391- 2397. |
| [21] | Anton de Groot C., Margo V., 2010, Formaldehyde-releasers in cosmetics in the USA and in Europe, Contact Dermatitis, 62: 221 - 224. |
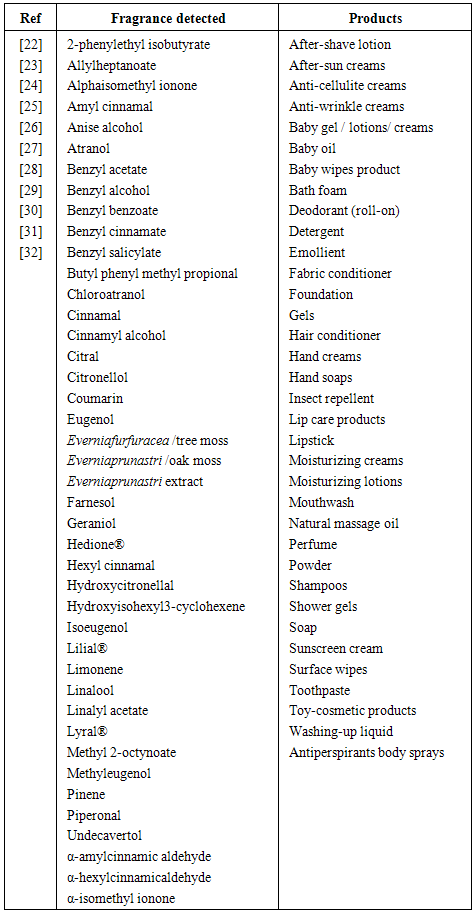
| [22] | Nardelli, A., Drieghe, J., Claes, L., Boey, L., Goossens, A., 2011, Fragrance allergens in ‘specific’ cosmetic products, Contact Dermatitis, 64: 212 - 219. |
| [23] | SCCS (Scientific Committee on Consumer Safety), opinion on fragrance allergens in cosmetic products, 13-14 December 2011. |
| [24] | Rastogi, S.C., Johansen, J. D., Menne, T., Frosch’, P., Bruze, M., Andersen, K. E., Lepoittevin, J. P., Wakelin, S., White, I. R., 1999, Contents of fragrance allergens in children’s cosmetics and cosmetic-toys, Contact Dermatitis, 41: 84 – 88. |
| [25] | Sanchez-Prado, L., Lamas, J.P., Alvarez-Rivera, G., Lores, M., Garcia-Jares, C., Llompart, M., 2011, Determination of suspected fragrance allergens in cosmetics by matrix solid-phase dispersion gas chromatography–mass spectrometry analysis, Journal of Chromatography A, 1218: 5055 – 5062. |
| [26] | Buckley, D.A., 2007, Fragrance ingredient labelling in products on sale in the U.K., British. Journal of Dermatology, 157: 295 – 300. |
| [27] | Villa, C., Gambaro, R., Mariani, E., Dorato, S., 2007, High-performance liquid chromatographic method for the simultaneous determination of 24 fragrance allergens to study scented products, Journal of Pharmaceutical and Biomedical Analysis, 44: 755 – 762. |
| [28] | Lamas, J.P., Sanchez-Prado, L., Garcia-Jares, C., Lores, M., Llompart, M., 2010, Development of a solid phase dispersion-pressurized liquid extraction method for the analysis of suspected fragrance allergens in leave-on cosmetics, Journal of Chromatography A, 1217: 8087 – 8094. |
| [29] | Rastogi, S.C., Johansen, J.D., Bossi, R. 2007, Selected important fragrance sensitizers in perfumes – current exposures, Contact Dermatitis, 56: 201 – 204. |
| [30] | Rastogi, S.C., Menne, T., Johansen, J.D., 2003 The composition of fine fragrances is changing, Contact Dermatitis, 48: 130 – 132. |
| [31] | Chen, Y., Begnaud, F., Chaintreau, A., Pawliszyn, J., 2006, Quantification of perfume compounds in shampoo using solid-phase microextraction, Flavour and Fragrance Journal, 21: 822 – 832. |
| [32] | Bossi, R., Rastogi, S.C., Bernard, G., Gimenez-Arnau, E., Johansen, J.D., Lepoittevin, J.P., Menn, T. A, 2004, Liquid chromatography-mass spectrometric method for the determination of oak moss allergens atranol and chloroatranol in perfumes, J. Sep. Sci., 27: 537 – 540. |
| [33] | Amit, S. C., Rekha B., Atul K. S., Sharad S. L., Dinesh K.C., Vinayak S. T., 2010, Determination of Lead and Cadmium in cosmetic products, Journal of Chemical and Pharmaceutical Research, 2(6):92-97. |
| [34] | Environmental Defence Canada, 2011, Heavy metal hazard, the health risks of hidden heavy metals in face makeup. |
| [35] | Ullah, H., Noreen, S., Ali Rehman, F., Waseem, A., Zubair, S., Adnan, M., Ahmad, I. 2013, Comparative study of heavy metals content in cosmetic products of different countries marketed in Khyber Pakhtunkhwa, Pakistan, Arabian Journal of Chemistry. |
| [36] | Nnorom, I.C., Igwe, J.C., Oji-Nnorom C.G., 2005, Trace metal contents of facial (make-up) cosmetics commonly used in Nigeria, African Journal of Biotechnology, 4(10): 1133 - 1138. |
| [37] | Sainio, E.L., Jolanki, R., Hakala, E., Kanerva, L., 2000, Metals and arsenic in eye shadows, Contact Dermatitis, 42: 5 - 10. |
| [38] | Volpe, M.G., Nazzaro, M., Coppola, R., Rapuano, F., Aquino, R.P., 2012, Determination and assessments of selected heavy metals in eye shadow cosmetics from China, Italy, and USA, Microchemical Journal, 101: 65 - 69. |
| [39] | Al-Saleh, I., Al-Enazi, S., 2011, Trace metals in lipsticks, Toxicological & Environmental Chemistry, 93(6): 1149 - 1165. |
| [40] | Ayenimo, J.G., Yusuf, A.M., Doherty, W.O., Ogunkunle, O.A., 2010, Iron, lead, and nickel in selected consumer products in Nigeria: A potential public health concern, Toxicological & Environmental Chemistry, 92(1): 51 - 59. |
| [41] | Gondal, M.A., Seddigi, Z.S., Nasr, M.M., Gondal, B., 2010, Spectroscopic detection of health hazardous contaminants in lipstick using Laser Induced Breakdown Spectroscopy, Journal of Hazardous Materials, 175: 726 - 732. |
| [42] | B. Bocca, G. Forte, F. Petrucci, A. Cristaudo, 2007, Levels of nickel and other potentially allergenicmetals in Ni-tested commercial body creams, Journal of Pharmaceutical and Biomedical Analysis 44: 1197–1202. |
| [43] | Liu, S., Hammond, S.K., Rojas-Cheatham, A., 2013, Concentrations and potential health risks of metals in lip products, Environmental Health Perspectives, 121(6): 705 - 710. |
| [44] | Piccinini, P., Piecha, M., Torrent, S.F., 2013, European survey on the content of lead in lip products, Journal of Pharmaceutical and Biomedical Analysis, 76: 225 - 233. |
| [45] | Ziarati, P., Moghimi, S., Arbabi-Bidgoli, S., Qomi, M., 2013, Risk Assessment of heavy metal contents (lead and cadmium) in lipsticks in Iran, International Journal of Chemical Engineering and Applications, 3(6). |
| [46] | Ababneh, F.A., Abu-Sbeih, K.A., Al-Momani, I.F., 2013, Evaluation of allergenic metals and other trace elements in personal care products, Jordan Journal of Chemistry, 8(3): 179 - 190. |
| [47] | Al-Qutob, M.A., Alatrash, H.M., Abol-Ola, S., 2013, Determination of different heavy metals concentrations in cosmetics purchased from the Palestinian markets by ICP/MS, AES Bioflux, 5(3). |
| [48] | Al-Saleh, I., Al-Enazi, S., Shinwari, N., 2009, Assessment of lead in cosmetic products, Regulatory Toxicology and Pharmacology, 54: 105 - 113. |
| [49] | Khalid, A., Bukhari, I.H., Riaz, M., Rehman, G., Ain, Q.U., Bokhari, T.H., Rasool, N., Zubair, M., Munir, S., 2013, Determination of lead, cadmium, chromium and nickel in different brands of lipsticks, IJBPAS, 2(5): 1003 - 1009. |
| [50] | Nnorom I.C., 2011, Trace metals in cosmetic facial talcum powders marketed in Nigeria, Toxicological & Environmental Chemistry, 93(6): 1135-1148. |
| [51] | SCCNFP (Scientific Committee on Cosmetic Products and Non-Food Products), Opinion of the scientific committee on cosmetic products and non-food products intended for consumers concerning salicylic acid, 4 June 2002. |
| [52] | Latorre, N., Silvestre, J.F. and Monteagudo. A.F., 2011, Allergic Contact Dermatitis Caused by Formaldehyde and Formaldehyde Releasers, Actas Dermosifiliogr, 102(2): 86–97. |
| [53] | Celeiro, M., Guerra, E., Lamas, J.P., Lores, M., Garcia-Jares, C., Llompart, M., 2014, Development of a multianalyte method based onmicro-matrix-solid-phase dispersion for the analysis of fragrance allergens and preservatives in personal care products, Journal of Chromatography A, 1344: 1 – 14. |
| [54] | Bridges, B., 1999, Fragrances and health Fragrance Products Information Network Environmental Health Perspectives, 107(7). |
| [55] | Norwegian Institute of Public Health, 2013, Preservatives - undesirable effects. http://www.fhi.no/eway/default.aspx? pid=240&trg=MainContent_6898&Main_6664=6898:0:25,8729:1:0:0:::0:0 &MainContent_6898=6706:0:25,8732:1:0:0:::0:0 (accesses on 5 May 2014). |
| [56] | U.S. Food and Drug Administration. Parabens, 2006, http://www.fda.gov/cosmetics/productsingredients/ingredients/ucm128042.htm (accesses on 27 September 2014). |
| [57] | Peregrino C. P., Moreno, M. V., Miranda, S. V., Rubio, A. D. and Leal, L. O., 2011, Mercury levels in locally manufactured Mexican skin-lightening creams, Int. J. Environ. Res. Public Health, 8: 2516 - 2523. |
| [58] | Wijnhoven, S.W.P., Ezendam, J., Schuur, A.G., van Loveren, H., van Engelen, J.G.M. Allergens in consumer products. RIVM Report 320025001/2008. |
| [59] | Adepoju-Bello, A. A., Oguntibeju, O. O., Adebisi, R. A., Okpala, N., Coker, H. A. B., 2012, Evaluation of the concentration of toxic metals in cosmetic products in Nigeria, Afri. J. Biotechnol, 11(97): 16360 - 16364. |
| [60] | SCCS (Scientific Committee on Consumer Safety), the SCCS's notes of guidance for the testing of cosmetic substances and their safety evaluation, 8th revision, 11 December 2012. |
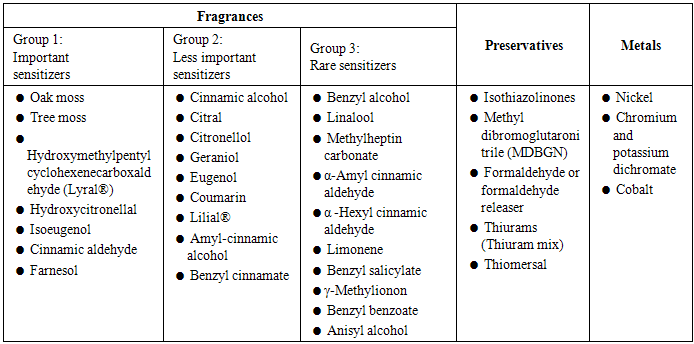
| [61] | Christensson, J.B., Matura, M., Gruvberger, B., Bruze, M. and Karlberg, A.T., 2010, Linalool – a significant contact sensitizer after air exposure, Contact Dermatitis, 62: 32–41. |
| [62] | Matura, M., Goossens, A., Bordalo, O., Garcia-Bravo, B., Magnusson, K., Wrangsjo, K. and Karlberg, A.T., 2002, Oxidized citrus oil (R-limonene): A frequent skin sensitizer in Europe, J Am AcadDermatol, 47(5): 709 – 714. |
| [63] | Darbre, P. D., Aljarrah, A., Miller, W. R., Coldham, N. G., Sauer, M. J. and Pope, G. S., 2004, Concentrations of parabens in human breast tumours, Journal of Applied Toxicology, 24: 5–13. |
| [64] | Onwordi C. T., Orizu C. O., Wusu A. D., Ogunwande I. A., 2011, Potentially Toxic Metals Exposure From Body Creams Sold In Lagos, Nigeria, Researcher, 3(1):30-37. |
| [65] | Al-Saleh, I., Al-Doush, I., 2007, Mercury content in skin-lightening cream and potential hazards to the health of Saudi women, Journal of Toxicology and Environmental Health: Current Issues, 51(2):123-130. |
| [66] | Al-Dayel, O., Hefne, J., AndAl-Ajyan, T., 2011, Human Exposure to Heavy Metals from Cosmetics, Oriental Journal of Chemistry, 27(1): 1-11. |
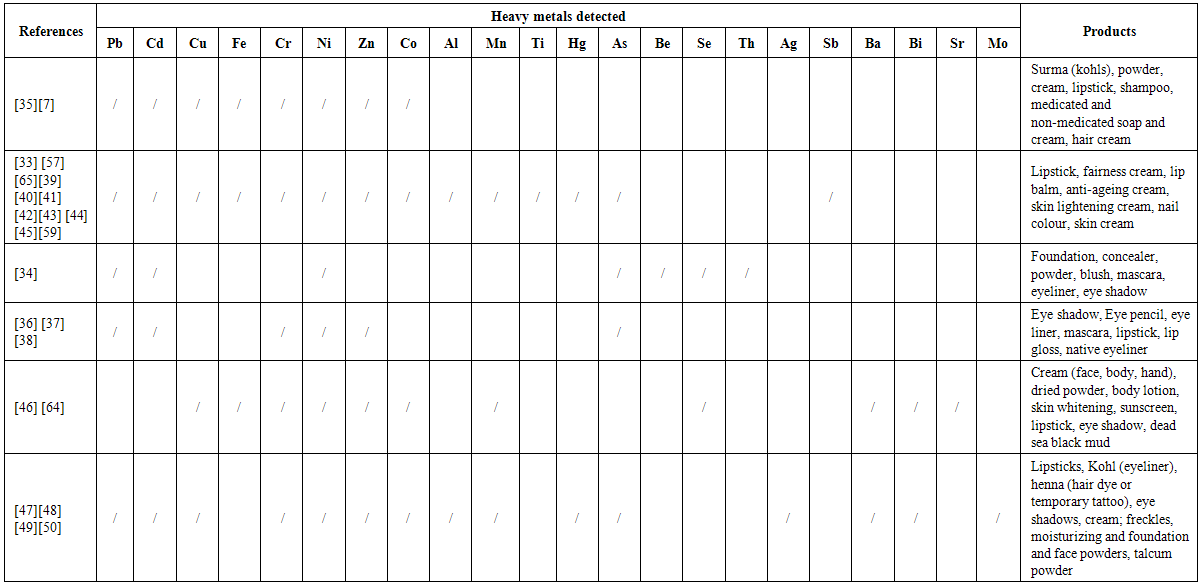
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML