-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Public Health Research
p-ISSN: 2167-7263 e-ISSN: 2167-7247
2014; 4(6): 219-224
doi:10.5923/j.phr.20140406.01
Malaria and Human Immunodeficiency Virus among Women Attending a Postnatal Clinic in Kenya
Rose Kakai1, 2, Lucia A. Odongo3, Ayub V. Ofulla2, 3, Richard Wachana4
1Department of Medical Microbiology, Maseno University, Maseno, Kenya
2Department of Biomedical Science & Technology, Maseno University, Maseno, Kenya
3Department of Public Health, Maseno University, Maseno, Kenya
4Department of Mathematics and Computer Science, University of Kabianga, Kericho, Kenya
Correspondence to: Rose Kakai, Department of Medical Microbiology, Maseno University, Maseno, Kenya.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
HIV reduces immunity leading to more frequent, prolonged and severe infections, while the effect of malaria enhances the progression of HIV to full-blown AIDS. Nyanza Province has a high HIV prevalence rate of 20 - 30%, and is also holoendemic for Plasmodium falciparum malaria transmission. These two diseases may be a major public health problem to postnatal women because there may still be an increased risk of malaria just like the pregnant period. The purpose of this study was to determine the prevalence of malaria among HIV positive and negative postnatal women attending Maternal and Child Health (MCH) clinic at a Health Centre in Kisumu, Nyanza Province. This was a cross-sectional study using simple random sampling to select 195 consenting post natal women. Semi structured questionnaires were used to elicit responses on demographic characteristics and mosquito bites prevention methods. Malaria parasite identification, HIV and haemoglobin (Hb) levels were determined by Field stain microscopy, Enzyme-linked Immunosorbent Assay (ELISA), and cyanmethaemoglobin Hb estimation technique respectively. Data was analyzed by Chi square, Fisher’s t-test and multiple logistic regression. Fifty (25.6%) women were positive for HIV and 27 (13.8%) for Plasmodium falciparum malaria. HIV positive women had a significantly higher prevalence of malaria than those HIV negative (30% vs 8.3%, OR = 4.75, 95% CI = 2.039 – 11.063, p =0.001). Those HIV positive with malaria had the lowest mean Hb level of 9.1 ± 1.2 g/dl. Mosquito bite prevention significantly reduced the prevalence of malaria irrespective of HIV status (p = 0.001). In conclusion, HIV infection was associated with increased prevalence of malaria and anaemia. More efforts to prevent malaria among HIV positive postnatal women may be an important intervention to reduce dual infections.
Keywords: Malaria, Human Immunodeficiency Virus, Postnatal women
Cite this paper: Rose Kakai, Lucia A. Odongo, Ayub V. Ofulla, Richard Wachana, Malaria and Human Immunodeficiency Virus among Women Attending a Postnatal Clinic in Kenya, Public Health Research, Vol. 4 No. 6, 2014, pp. 219-224. doi: 10.5923/j.phr.20140406.01.
Article Outline
1. Introduction
- Malaria and Human Immunodeficiency Virus (HIV) both cause substantial morbidity and mortality, particularly among pregnant women in sub-Saharan Africa. Primigravida tend to be more susceptible than multigravida, as women gain immunity against malaria during successive pregnancies, particularly in areas of high transmission [1]. The risk for malaria may not return to pre-pregnancy levels immediately after delivery [2].HIV positive pregnant women are more at risk of malaria compared to HIV negative pregnant women [3], [4]. HIV infects CD4 helper lymphocytes, which are responsible for the initiation of nearly all immunological responses to pathogens, particularly the cell mediated immunity [5], [6]. Since HIV infection results in immune deficiency, it may alter the ability of the infected individual to mount proper immune responses against malaria parasites. Acute malaria episodes temporarily increase virus replication and hence HIV viral load, and enhance the progression of HIV towards full-blown AIDS [6], [7]. Recrudesce of malaria may be more frequent because of the immune suppression normally experienced by pregnant women [5]. Current evidence suggests an effort should be made to detect and cure malaria during pregnancy so that women do not enter the postpartum period with residual parasites [3]. Malaria has been undergoing a dramatic resurgence in recent years due to increasing resistance of the malaria parasite to affordable drugs, hampering the efforts to control this debilitating disease. Since 2002, several countries started scaling up free of charge or highly subsidized provision of insecticide treated nets (ITNs) for children under 5 years old and pregnant women but this has rarely been extended to postnatal period [3]. In Kenya’s national policy, intermittent preventative treatment with sulfadoxine–pyrimethamine (IPT-SP) is used for the control of malaria in pregnancy. Postpartum women are encouraged to share ITNs that are provided for children aged under 5 years. Depending on the timing of the last dose in pregnancy, drug half-life and level of drug resistance, protection could be prolonged into the early postpartum period and may result in an underestimation of the postpartum susceptibility to malaria.Although pregnant women are more susceptible to malaria than their non-pregnant counterparts, less is known about the risk of malaria in the postpartum period [3]. There may still be an increased risk of malaria in the postpartum period. Studies done on HIV in relation to malaria among women have mainly been concerned with the antenatal period. The purpose of this study was to determine the prevalence of malaria among HIV positive and negative women attending postnatal services at a clinic in Kisumu town. Results are likely to enhance the prevention of malaria in HIV positive mothers during the postnatal period.
2. Methods
2.1. Study Area
- The study was undertaken at a Health Centre in Nyalenda sub-location, West Kolwa location, Winam division in Kisumu District of Nyanza Province, Western Kenya. At the time of the study, Kisumu town had a population of approximately 300,000 and the study Health Centre serves about 25,406 people.
2.2. Study Design and Population
- This was a descriptive cross-sectional study of 195 out of 700 women attending postnatal MCH services during the study period at a Health Centre in Kisumu town. The sample size was determined as described by Mugenda and Mugenda [8], based on 22% prevalence of HIV and malaria dual infection among pregnant women in Kisumu [9] and adjusted for a population less than 10,000.
2.3. Sampling Procedure
- A semi-structured questionnaire was used to obtain data on socio-demographics and mosquito bite prevention methods from 195 primigravida women with children aged 12 months or below, who gave informed consent to participate. As part of the routine Health Centre services, all the women received Intermitent Prophylaxis Treatment (IPTp) for malaria during pregnancy, and were counseled by trained MCH clinic staff before collection of 5ml peripheral blood using a sterile needle and syringe for HIV and Hb testing. All HIV positive women were referred by MCH staff to Patient support centre for ARV treatment and care. An aliquot of the blood was used to prepare thick and thin smears for malaria parasites microscopy. Approval to conduct the study was obtained from Maseno University School of Graduate Studies and Medical Officer of Health, Kisumu. All information collected was confidential.
2.4. Laboratory Procedures
2.4.1. Detection of HIV Antibody
- DetermineTM (Abbott, Tokyo, Japan) and UnigoldTM (Trinity Biotech plc. Ireland, UK),immune-chromatographic tests for the qualitative detection of antibodies for HIV 1 and HIV 2 were used for screening and confirmation, respectively. Upto 2 drops of a blood sample were added to a sample pad using a dropper and left to absorb into the pad. One drop of Chase buffer was applied to the sample pad and left for 15 to 20 minutes before reading the results.
2.4.2. Identification of Malaria Parasites
- For each participant, a thick blood smear was made on a microscope slide and left to dry after which it was stained with field stain A (Methylene blue and Azure) for about 15 seconds then rinsed with distilled water. The smear was again stained with Field stain B (Eosin) and left for about 15 seconds. It was rinsed with distilled water, left to dry and examined for presence of Plasmodium trophozoites under oil immersion at X100 magnification. A trained technician examined the smears for the presence of malaria parasites and identified the species based on the appearance of trophozoites and gametocytes. All slides were counter-checked by a second technician. Malaria infection was defined as the presence of parasites in thick or thin smears, independent of the presence or absence of clinical signs and symptoms.
2.4.3. Determination of Haemoglobin Levels
- This was done using cyanmethaemoglobin Hb estimation technique. Haemoglobin is oxidized to methaemoglobin by potassium ferri-cyanide. Methaemoglobin in turn combines with potassium cyanide to form cyanmethaemoglobin. Briefly, 5ml of Drabkin’s solution (SIGMA, Missouri, USA) containing potassium fericyanide and sodium cyanide was put in a test bottle and 20µl of blood sample added using a clean pipette. After mixing well, it was left to stand for 5 to 10 minutes for colour development and a colorimeter used to make the readings. Drabkin’s solution was used as a blank to determine the zero reading of the colorimeter, after which the mixture was put in the machine. The absorbance of the solution in the colorimeter was read using filter number 540 nm. The readings from the colorimeter were matched with Hb levels from the calibration curve. Women with Hb level < 7 g/dl were considered to be severely anaemic (normal range for adult females: 12- 16 g/dl).
2.5. Data Analysis
- Data was analyzed using statistical packages for social sciences (SPSS) version 16 (SPSS for Windows, Version 16.0. Chicago, SPSS Inc. Released 2007). Differences in proportions of discrete and continuous variables were statistically compared using chi-square test and Fisher’s t-test respectively. Multiple logistic regression and odds ratios (OR) were computed with 95% confidence interval (CI) level to measure the strength of associations. A p-value < 0.05 was considered significant.
3. Results
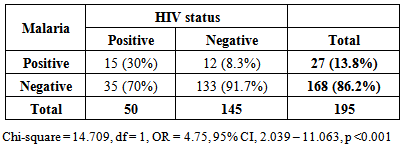
- Results in Table 1 shows that the prevalence of malaria was significantly higher in HIV positive than negative women (15/50; 30% vs. 12/145; 8.3%, OR = 4.75, 95% CI, 2.039 – 11.063, p < 0.001). This indicates that HIV positive women were 5 times more likely to have malaria than those who were HIV negative. Overall, 27 (13.8%) women tested positive for Plasmodium falciparum malaria parasites.
|
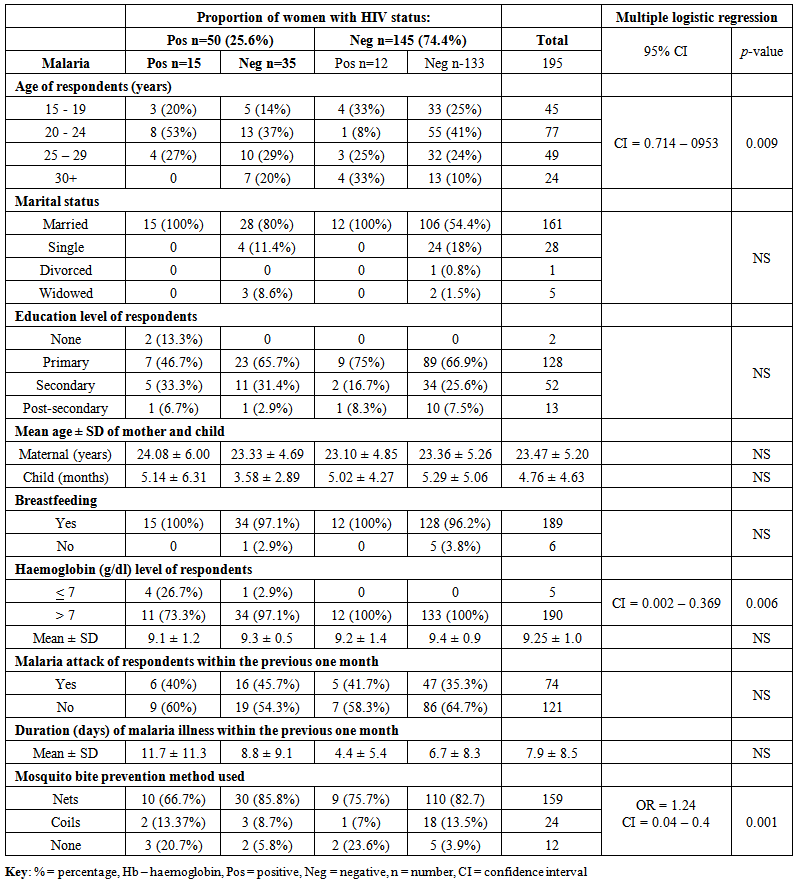 | Table 2. Demographic and clinical characteristics in relation to HIV and malaria |
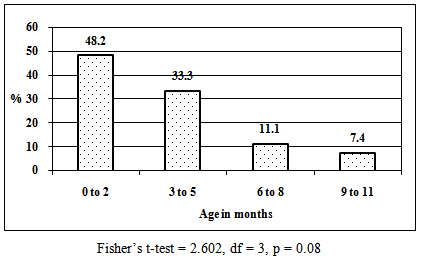 | Figure 1. Age distribution of children whose mothers had malaria (n = 27) |
4. Discussion
- Overall HIV sero-prevalence among postnatal women in this study was 25.6%. Previous reports have indicated that 24.8% of pregnant women in Kisumu District are HIV positive [10]. The highest prevalence of both HIV and malaria co-infections was in the age group 20 – 24 years and this declined with increasing age. Other studies indicate that HIV positive pregnant women are more at risk of malaria compared to HIV negative pregnant women suggesting that there may still be an increased risk of malaria in the postpartum period, just like the pregnant period [3], [4]. In our study, prevalence of malaria among HIV positive and negative postnatal women was 30% and 8.3% respectively. These findings suggest that increased prevalence of malaria in HIV infection during postnatal period may probably be due to a spillover effect of pregnancy related immunosuppression as factors that protect women against malaria after delivery may take much longer to normalize [11], [12], and might extend beyond the usual limit of the postpartum period which is defined as the period until six weeks after delivery [13]. Further analysis revealed that there was an inverse relationship between malaria prevalence and age of the children. Malaria prevalence among participants declined as age of the children increased, with the highest prevalence occurring among women whose children were aged 2 months and below. Whether this was due to pregnancy related spillover effect regarding lowered resistance into the postnatal period [2] or women with younger children woke up more frequently during the night to feed their infants thereby exposing themselves to mosquito bites is unknown. It was noted that all women who had malaria were breastfeeding. A study in Kenya found that breastfeeding HIV positive mothers had higher mortality [14], while other studies were on the contrary [15], [16]. However, breastfeeding should be encouraged in resource poor settings to ensure that as many infants as possible benefit from proper nutrition [17].Dual malaria and HIV is associated with poor clinical outcome. There is overlapping immune reactivity in blood containing HIV antigens and that with P. falciparum antigens [18]. Immune response to malaria can increase the pool of lymphocytes available for HIV infection, resulting in accelerated progression to AIDS. The effect of dual infection is greater in non-pregnant women with advanced HIV disease and a suppressed immune function. In our study, information regarding HIV disease progression and immune function status were not determined. In areas of stable malaria, transmission is intense and continuous, thus HIV immunosuppression may increase rates of malaria infection and clinical malaria disease [19]. HIV infection increases the incidence of P. falciparum parasitaemia and is associated with the development of severe malaria, anaemia, cerebral malaria, and high parasite density [20]. On the contrary, other workers argue that there is no association between HIV and the presence or degree of malaria parasitemia [21], [22]. Malaria is usually accompanied by haemolysis and is an important cause of fever, convulsions, anaemia, and death. Anaemia, even when mild, is associated with reduced work productivity [23]. Women's self-reported morbidity frequently cites symptoms in the postpartum period that could be suggestive of, or lead to anaemia, including chronic fatigue and excessive bleeding. Both HIV and malaria may contribute independently to anaemia. In severe or prolonged attacks of malaria, anaemia may be profound, and lead to blood transfusion, which is a potential risk factor for HIV infection. In the present study, all the women who were anaemic were HIV positive, 80% of whom had malaria, suggesting that anaemia was associated with HIV and malaria co-infections. In a previous study in Kisumu, dual infection with HIV and malaria more than doubled the risk of moderate to severe anaemia (Hb < 8 g/dl) in both primigravidae and multigravidae [9]. In Dar-es-salaam, Tanzania, 49% of parous non-pregnant women were mild (Hb < 12 g/dl) and 1.6% severely anaemic (Hb < 7 g/dl) [24]. In our study, 2.6% of postnatal women had severe anaemia ((Hb < 7 g/dl)) and this prevalence rose to 26.7% in HIV and malaria dual infected women. Thus anaemia is likely to be a public health problem where HIV infection and malaria are both common [23]. Malarial antigens and pigments released during the burst of red blood cells stimulate cytokines that can activate HIV replication [25]. Tumour necrosis factor (TNF alpha) is a cellular signalling protein or cytokine released in response to anti-malarial immune activation, and it is associated with increased rates of HIV replication. However, investigators in Malawi found that during SP malaria treatment, blood levels of TNF alpha and HIV decreased [7]. This adds weight to the suggestion that suppressing malarial infection may result in a lowered HIV viral burden [25].Kenya has boosted the deployment of insecticide treated bed nets (ITBNs). Despite the highly subsidized provision of ITBNs for children under five years and pregnant women, some women were either unable to purchase them due to financial constraints, or the nets were not available at the distribution points. A much less emphasized part of the WHO Roll Back Malaria recommendation on personal protection [26], is that women in the postpartum period should be encouraged to use ITNs [3]. There are no other recommended preventive strategies for postpartum women. This is despite malaria being one of the leading causes of hospital admission and maternal death among postpartum women in Zambia [2] and India [27]. In our study, mosquito bite prevention was protective against malaria irrespective of the maternal HIV serostatus.
5. Conclusions
- HIV seropositive postnatal women had higher prevalence of malaria and severe anemia. To maximize public health benefits, prevention of malaria infection in postnatal women should be enhanced irrespective of HIV serostatus. The association between age of the child and malaria among the women needs further investigation.
ACKNOWLEDGEMENTS
- We thank the following for making this work possible; Maseno University, Health Centre staff and all the women who accepted to participate in the study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML