-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Public Health Research
p-ISSN: 2167-7263 e-ISSN: 2167-7247
2013; 3(3): 50-53
doi:10.5923/j.phr.20130303.04
Breeding Sites of Anopheles gambiae s. l. (Gilles) in Rural Lowland Rainforest, Rivers State, Nigeria
M. Aline E. Noutcha, Samuel N. Okiwelu
Entomology and Pest Management Unit, Department of Animal and Environmental Biology. University of Port Harcourt, Port Harcourt, Nigeria
Correspondence to: Samuel N. Okiwelu, Entomology and Pest Management Unit, Department of Animal and Environmental Biology. University of Port Harcourt, Port Harcourt, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Studies were undertaken in the rainy season, May-September, 2009, to determine the breeding sites of the major malaria vectors in four villages (Omanwa, Ipo, Omademe, Ubima) in the Ikwerre Local Governement Area (LGA), Rivers State, Nigeria. A 100ml-ladle and Pasteur pipette were used for collections in large volume of water and Phytotelmata respectively. Standard keys were used for identification. A total of 979 immatures (larvae and pupae) were collected from 3 general breeding site- types: “Pools” (open-lit drainage, Puddle, Depression caused by tyre marks), Containers (plastic and metal) and Phytotelmata (Leaf Axils and depressions on felled trees). The 3 general site-types (“Pools”, Containers, Phytotelmata) yielded 674, 280 and 25 immatures respectively. Numbers of immatures per site, in the three categories were “Pools” (19.47/site), Containers (36.77/site) and Phytotelmata (0.60/site); these differences were significant (F=3.58; df=1,12; p<0.05). Percent frequencies of immature occurrence in relation to numbers examined in the general site-types were, “Pools” (73.68%), Containers (32.25%) and Phytotelmata (21.43%). Frequency differences were significant (F=3.04; df: 1,2=3.02, p<0.05). These results are compared to those from other studies and discussed within the context of the behavioural plasticity of An. gambiae s.l. An. gambiae s.l. predominantly utilized the classical breeding sites, but atypical sites were also used.
Keywords: Anopheles Gambiae s.l., Breeding Sites, Malaria, Rural Rainforest, Nigeria
Cite this paper: M. Aline E. Noutcha, Samuel N. Okiwelu, Breeding Sites of Anopheles gambiae s. l. (Gilles) in Rural Lowland Rainforest, Rivers State, Nigeria, Public Health Research, Vol. 3 No. 3, 2013, pp. 50-53. doi: 10.5923/j.phr.20130303.04.
Article Outline
1. Introduction
- A recent review concluded that about 1 million deaths (range 744,000-1,300,000) from the direct effects of malaria occur yearly in Africa, more than 75% of them in children[1]. Malaria accounts for 30% of all childhood deaths and 11% of maternal deaths in Nigeria[2]. Significant variations in vector biology within and between countries have been reported in malaria epidemiological studies[3]. It is now generally agreed that a clear understanding of the detailed epidemiology of the disease is a pre-requisite to effective malaria control in the African sub-region[4].A number of factors have contributed to the remarkable success in the reduction of the malaria burden in four countries: Brazil, Eritrea, India and Vietnam[5]. These include, a targeted technical approach using a package of effective tools and data-driven decision-making. An Integrated approach to Vector Management (IVM) has been recommended by the World Bank[5]: useofInsecticide- Impregnated Bed Nets, Indoor Residual Spray (IRS) and Larviciding. An important component of larviciding is an accurate knowledge of the breeding sites of major malaria vectors. Studies to identify the breeding sites of Anopheles gambiae s.l. were therefore undertaken, April-September, 2009 in five villages (Ipo, Omanwa, Omademe, Ubima, Ozuaha) in the Ikwerre Local Government Area (LGA), Rivers State. This was to complement the Indoor Residual Spray (IRS), being undertaken simultaneously.
2. Materials and Methods
2.1. Study Area
- The four villages are located in the lowland rainforest: Ipo (06º57.5´N, 05º02.3´E), Omanwa (06º53.5´N, 05º03.8´E), Omademe (06º575N, 05º05.1´E), Ozuaha (06º55.3´N, 05º03.5´E) and Ubima (06º54.2´N, 05º07.4´E). There are two overlapping seasons: April-September (rainy) and October-March (dry). The main occupation of the local people is farming, but some are involved in wildlife hunting and bushmeat trade.
2.2. Methods
- Randomly selected grids (100m x 100m) were surveyed in each village. All possible ground level larval habitats and phytotelmata were identified in each grid and the type of habitat noted. Larvae were collected in the mornings on weekdays during the period, April-September, 2009. A 100ml-laddle and Pasteur pipette were the main sampling tools. Immatures were transported daily to the laboratory for identification, using standard keys[7-8]. During the preliminary study, collected immatures were reared to adults and subsequently identified by several keys[6-8] to confirm that they were those of An gambiae s.l.
3. Results
3.1. General Breeding Site-types
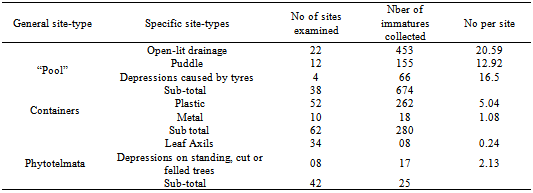
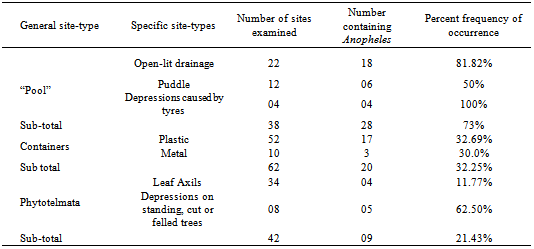
- A total of 979 immatures (larvae and pupae) of An. gambiae s.l. were collected. These were collected from 3 general site-types: “Pools” (Open-lit drainage, Puddle, Depression caused by tyre marks); containers (Plastic, Metallic) and Phytotelmata (Leaf Axils, Depressions on standing, cut or felled trees). Sizes of containers varied, 4.0-20.0L. The 3 general site-types: “Pools”, Containers, Phytotelmata) yielded 674, 280 and 25 immatures respectively. These differences were significant (F= 15.46; df 2,3=9,55; P<0.05) (Table 1). The numbers of immatures per site in each general site-type were: “Pools” (19.47/site), Containers (36.77/site) and Phytotelmata (0.60/site); these differences were significant (F=3.58; df 1,12= ; P<0.05) (Table 1). Percent frequencies of occurrence of immatures in relation to numbers of sites examined in each general site-type were: “Pools” (73.68%), Containers (32.25%) and Phytotelmata 21.43%) (Table2).
|
|
|
3.2. Distribution within “Pools” Category
- In the “Pools” category the distribution of immatures was: open-lit drainage (453), Puddle (155) and depression caused by tyres (66); these differences were significant (F=3.04; df 1,2=3.02; P<0.05). The numbers of immatures per site in these sub-categories were: open-lit drainage, 20.59/site; puddle, 12.92/site and depressions caused by tyres, 16.5/site (Table1).
3.3. Distribution within Container Category
- More immatures were collected from plastic (262) than metal (18) containers. The numbers per site were 5.04/site (plastic) and 1.08/site (metal); these differences were significant (F=1.85; df 1,2= 1.20; P<0.05).
3.4. Distribution within Phytotelmata
- Immatures were distributed almost equally between Leaf Axils (04) and Depressions on standing, cut or felled trees (05); the frequencies of occurrence were quite disparate: Leaf Axils (11.77%) and Depressions on standing, cut or felled trees (62.50%).
3.5. Variation among Communities
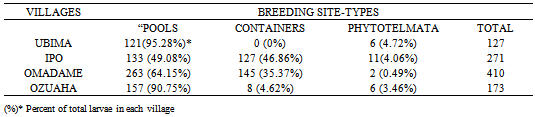
- Numbers of immatures collected from “pools” at Ubima and Ozuaha were more than 90% of total collection in each village, but these declined to 64.15% and 49.08% at Omademe and Ipo respectively. The numbers from containers rose from 4.62% at Ozuaha to 35.37% and 46.86% at Omademe and Ipo respectively (Table 3).
4. Discussion
- Parental capacity to enhance offspring survivorship and fecundity contributes, by definition, to parental fitness. In organisms with no parental care or juvenile dispersal, offspring survival and growth may depend strongly on the quality of the habitat in which they are deposited. Thus, when potential habitats vary in their suitability for juveniles, females are expected to choose habitats that maximise their fitness[9]. In mosquitoes, such oviposition habitat selection has been demonstrated in response to physical and chemical suitability for larval development[10], habitat size and resource availability[11-12], the presence and density of conspecific competitors[13] and the presence of predators [14]. The absence of An. gambiae s.l. immature from the randomly selected grid at Omanwa, highlighted the permanence of the colonisation of rural areas by Cx quinquefasciatus.Earlier workers described the typical breeding habitats of freshwater An. gambiae s.l. as shallow sun-lit pools, such as burrow pits, drains, brick pits, car tracks, ruts, hoof prints around ponds and wells. Nearly 70% of larvae from this study were collected from these classical breeding sites. According to[9] Kitflawi et al., these sites maximize the fitness of An. gambiae s.l.However, other authors observed An. gambiae s.l. breeding in vegetative pools, irrigation canals, swamp edges and permanents wells[6]. Although Okorie[15] observed that An. gambiae s.l. was least attracted to containers, approximately 30% of all immatures were collected from containers and at Omademe and Ipo, the percents from containers rose to 35% and 47% respectively. These results indicate the behavioural plasticity of An. gambiae s.l. in the selection of breeding site. Plasticity in An. gambiae s.l. had been discussed extensively by White[16]. The low number of immatures in phytotelmata is not surprising because they had never featured as preferred breeding sites of An. gambiae s.l., probably because of limited size and resource availability[11-12]. Although the frequencies of occurrence of immatures in metal and plastic containers were almost identical, the higher numbers of immatures and numbers per sites in plastic containers were probably associated with variation in physical and chemical suitability of media for larval development[10].
5. Conclusions
- Based on the total numbers and frequency of occurrence of immature, Anopheles gambiae s.l. predominantly utilized the classical breeding sites (Open-lit drainage, puddles, depressions caused by tyre marks); atypical breeding sites (plastic and metal containers, phytotelmata) were also used. These observations highlighted the plasticity of An. gambiae s.l.
ACKNOWLEDGEMENTS
- This study was undertaken during a World Bank-funded project on the Indoor Residual Spray (IRS)-Integrated vector Management (IVM) for malaria control. The beneficiary was the Rivers State Ministry of Health, Port Harcourt, Nigeria. We are indebted to village chiefs, who granted us access to the study sites and the youths that served as guides in each of the villages. The commitment of Messrs Pasiya Otufu, Stephen Ozule and Ibrahim Sow Kamara during field work is appreciated. The assistance on statistical analyses by Mr Bukola Amoo Oyebade of the Forestry Biometrics and Measurement Unit, Department of Forestry and Wildlife Management, Faculty of Agriculture, University of Port Harcourt is gratefully acknowledged.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML