-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Public Health Research
p-ISSN: 2167-7263 e-ISSN: 2167-7247
2013; 3(2): 18-23
doi:10.5923/j.phr.20130302.02
Interference of Extracts Obtained from Baccharis Coridifolia D. C. (Asteraceae-Astereae) Action of Antibiotics
Sideney Becker Onofre, Marilde Canton, Paula Andressa Pires
Department of Biological Sciences and Pharmacy, FAED - Faculty of Educational of Dois Vizinhos - União de Ensino do Sudoeste do Paraná – UNISEP - Av. Presidente Kennedy, 2601 - Bairro Nossa Senhora Aparecida - Dois Vizinhos - Paraná – Brazil
Correspondence to: Sideney Becker Onofre, Department of Biological Sciences and Pharmacy, FAED - Faculty of Educational of Dois Vizinhos - União de Ensino do Sudoeste do Paraná – UNISEP - Av. Presidente Kennedy, 2601 - Bairro Nossa Senhora Aparecida - Dois Vizinhos - Paraná – Brazil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The family Asteraceae is the most numerous group within the Angiosperms. Plants from this family are studied for their chemical composition and biological activity. In these studies, the allelopathic, antimicrobial, cytotoxic and anti-inflammatory effects are highlighted. The aim of this study was to evaluate the action of the aqueous (AF1) and the oily (OF2) fractions obtained from the ethanol extract of Baccharis coridifolia D. C. on the antimicrobial activity of antibiotics used in clinical treatment. The drugs chloramphenicol (30 µg), norfloxacin (10 µg), sulfamethoxazole / trimethoprim (75/23 µg), vancomycin (30 µg) and amoxicillin (10 µg) were saturated with the fractions AF1 and OF2, and used pure for further comparison. The strains Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923 were used. The results showed that both fractions interfered on the activity of the antibiotics tested. Synergistic, antagonistic or indifferent behavior was observed in the interactions, with various degrees of susceptibility to the antibiotics tested. These results show that the use of products derived from plants can, in some cases, interfere with the effectiveness of antibiotics during clinical therapy.
Keywords: Baccharis, Antibiotics, Metabolites, Biological Activity
Cite this paper: Sideney Becker Onofre, Marilde Canton, Paula Andressa Pires, Interference of Extracts Obtained from Baccharis Coridifolia D. C. (Asteraceae-Astereae) Action of Antibiotics, Public Health Research, Vol. 3 No. 2, 2013, pp. 18-23. doi: 10.5923/j.phr.20130302.02.
Article Outline
1. Introduction
- Synthetic products started to appear more frequently in pharmacological treatment after the Industrial Revolution and the development of organic chemistry. Obtaining pure compounds became easier, with the development of structural change processes to produce safer and more active medicaments, and also because of the growing economic power of the large pharmaceutical companies. Even so, natural products have not lost their place in therapy, being considered by the population as safe medicines, as 25% of drugs prescribed worldwide are of natural origin[1].The Asteraceae family is the most numerous systematic group within the Angiosperms, as it comprises about 1,100 genera and 25,000 species. These plants are extremely varied in appearance, mainly including small herbs or shrubs, and rarely trees[2]. About 98% of the genera are small plants, which are found in all types of habitats, but mainly in the mountainous tropical regions of south America[3].Plants in this family are extensively studied for their chemical composition and biological activity, with some supporting the development of new drugs, insecticides and other products[2,4,5].The genus Baccharis (tribe Astereae) is represented by over 500 species largely distributed in the higher regions of Brazil, Argentina, Colombia, Chile and Mexico. In Brazil, 120 species of Baccharis have been described, with most of them located in the Southeast[6,7].Baccharis species are generally shrubs and are in average 0.5 to 4 m tall. They have a high socio-economic value, with a wide dispersion in the Brazilian states of Santa Catarina, Paraná, São Paulo, Rio Grande do Sul, and other regions of the country. These plants are mainly consumed as teas, with indications for stomach, liver and prostate problems, as well as anemia, inflammations, diabetes, and body detoxification. In Brazil and Argentina, for example, B. crispa and B. coridifolia are used to heal wounds and inflammations. B. trimera, B. uncinella and B. articulata are also well-known in alternative medicine. B. genistelloides is a medicinal herb widely used in Brazil for a variety of diseases, including digestive and liver disorders, malaria, ulcers, diabetes, anemia, diarrhea, urinary tractinflammations, tonsillitis, verminosis and Hansen's disease[8].Phytochemical studies on Baccharis species have reported the presence of flavonoids, diterpenes and triterpenes, as well as a greater accumulation of flavones, flavonols and labdane and clerodane diterpenes[2-10].About 120 Baccharis species have been studied chemically, with 30 of them investigated for biological activity. Generally, the most common compounds are the flavonoids and labdane and clerodane diterpenes, although kaurane diterpenes, triterpenes, germacrene, coumaric acids, trichothecene, sesquiterpenes and phenylpropanoids are also frequently found. Allelopathic, antimicrobial,anti-inflammatory and cytotoxic effects are highlighted in biological activity studies. The most researched species for chemical composition and/or biological activity include B. megapotamica, B. incarum, B. trimera, B. trinervis, B. salicifolia, B. crispa, B. coridifolia, B. dracunculifolia, B. uncinella, B. grisebachii and B. tricuneata[2-10].The flavonoids quercetin, apigenin, hispidulin and eupatorin have already been isolated from B. coridifolia. In this species, the flavonoid rutin is higher in plants harvested during summer, when compared to plants harvested in winter and spring[11].Some medicaments are made from flavonoids, in particular for the treatment of circulatory diseases and hypertension, acting as a vitamin C cofactor. Other studies suggest that some flavonoids show considerable antitumor action, and may also have antiviral, anti-hemorrhagic, hormonal, anti-inflammatory, antimicrobial and antioxidant effects[12].Four flavonoids were isolated from the methanol extract of B. coridifolia leaves: 5,4'-dihydroxy-7-methoxy-flavone (genkwanin),5,4'-dihydroxy-6,7-dimethoxy-flavone(cirsimaritin), 5,7,4'-trihydroxy-flavone (apigenin) and5,7,4'-trihydroxy-6-methoxy-flavone (hispidulin). These compounds showed antimutagenic activity, indicating that they are responsible for such activity in the B. coridifolia[8]. Studies demonstrate that B. coridifolia has a potential antiophidic effect, inhibiting proteolytic activity, hemorrhage, myotoxicity and edema induced by the venom of some Bothrops species[13-15].The term "drug interaction" refers to the interference of one drug on the action of another, or of a food or nutrient on the action of a drug. It is important to remember that some drug interactions are beneficial or desirable in the treatment of concomitant diseases, reducing adverse effects and prolonging desired effects, preventing or delaying bacterial resistance, increasing adherence to treatment, increasing efficiency or enabling dose reduction. The undesirable interactions are linked to a reduced drug action or increased contrary results, incidence and range of adverse effects, as well as therapy costs, without increasing the therapeutic benefit. Interactions that lead to a reduced drug activity and, therefore, a loss in effectiveness, are difficult to detect and may be responsible for therapy failure or disease progression. Age, general health, kidney and liver function, alcohol consumption, smoking, diet, as well as genetic and environmental factors influence the susceptibility to drug interactions[16].B. coridifolia (Figura 1) products are widely marketed in Brazil, and are being consumed in an irrational and indiscriminate manner together with other drugs,[17]. In this context, this study aims to evaluate the effects of B. coridifolia ethanol extract on the antimicrobial activity of antibiotics used in clinical therapy.
 | Figure 1. Baccharis coridifolia D. C. – Asteraceae-Astereae |
2. Material and Methods
2.1. Collection Site
- The plant material was collected in the countryside in the town of Dois Vizinhos, Paraná, Brazil (25°46’23’’S, 53°03’15’’; elevation 620m). The specimens were identified and stored in the collection at União de Ensino do Sudoeste do Paraná – UNISEP, Campus of Dois Vizinhos, PR. When the collections arrived at the laboratory, material was selected by separating the leaves, trunk and roots, which were dried in a stove for seven days at 60ºC ±4. The material was then ground and stored in paper bags in a dry environment. All of the collections were authorized by IBAMA under the permit number13.234-2 (1 August, 2006).
2.2. Extraction by Maceration of the Plant Material
- The 30% (w/v) hydroalcoholic extract was prepared by the maceration and trituration method, using a 70% ethanol solution as the extractor. The extract was filtered in absorbent cotton pads. After extraction, the material obtained was stored in its concentrated form. Two fractions were obtained: a water-soluble fraction (aqueous fraction or AF1) and a fraction soluble in organic solvent (oily fraction or OF2). Both fractions were stored in amber bottles for further analyses.
2.3. Antimicrobial Activity of the Fractions
- The method used was based on the disk diffusion susceptibility test. In this method, a standardized solution of a specific microorganism is inoculated on agar plates. Then, paper disks impregnated with the substances investigated for antimicrobial activity are placed on the agar. Such substances diffuse in the culture medium and, if the sample in question exhibit inhibitory activity against the microorganism, a clear ring is formed around the disk. After the incubation period, and under the specific conditions for the microorganism development, inhibition zones are measured around each disk[18-21].
2.4. Determination of the Minimum Inhibitory
2.4.1. Concentration Index (MIC)
- The bacterial suspensions of E. coli ATCC 25922 and S. aureus ATCC 25923 were inoculated onto plates containing Mueller-Hinton agar, with a Drigalski spatula. After this procedure, Whatman no.1 filter paper disks (6 mm diameter) previously prepared were transferred to the plates. The antibiotic disks, with and without the added extracts of B. coridifolia, were evaluated on each plate. Subsequently, the plates were incubated at 35 ± 1 °C for 24 h. After this period, the plates were analyzed for the presence of inhibition zones (measured in mm). All assays were performed in triplicate. Inhibition zone diameters were interpreted according to the Clinical and Laboratory Standard Institute, recommended guidelines[22].
2.4.2. Preparation of Paper Disks
- The fractions obtained were used for preparing disks of antimicrobial activity. The AF1 was dissolved in water, whereas OF2 was dissolved in DMSO (Dimethyl sulfoxide) to achieve concentrations of 100, 50, 25, 12.5, 6.25 and 3.12 mg/liter.Two sets of disks were prepared with the obtained concentrations. The first set of disks were prepared with Whatman filter paper no.1 (6 mm diameter), saturated in AF1 and OF2, according to the previously mentioned concentrations. This procedure aimed to evaluate the antimicrobial activity of the aqueous and oily fractions and determine the Minimum Inhibitory Concentration (MIC).The second set of disks was obtained commercially (Newprov). These disks already contain a determined concentration of each antibiotic.Chloramphenicol (30 μg), norfloxacin (10 μg), sulfamethoxazole/trimethoprim (75/23 μg), vancomycin (30 μg) and amoxicillin (10 μg) disks were saturated with both fractions by complete immersion in the dilutions, whose MIC had been determined. Subsequently, these disks remained in a laminar air flow system over 2 h for evaporating the excess solvent. Disks containing only antibiotics, without the oily and aqueous added fractions, were used as controls.
2.4.3. Preparation of the Culture Medium
- The culture medium used for testing the antimicrobial activity was the Mueller-Hinton agar (MH - Merck), prepared from the dehydrated medium, according to the manufacturer's recommendation[18].
2.4.4. Preparation of The Inocula
- E. coli ATCC 25922 (beta-lactamase negative) and S. aureus ATCC 25923 were used. The strains were reconstituted according to supplier recommendations. The microbial culture was standardized at 108 cells/mL, which is compared to the 0.5 McFarland turbidity standard[23].
2.5. Benchmarks for Assessment
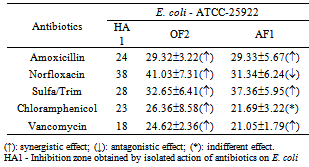
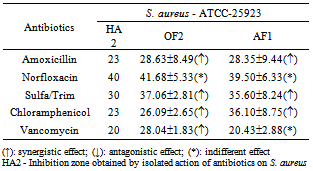
- CLSI guidelines[21] were used to verify the antimicrobial susceptibility of the ATCCs. Synergistic effect was considered when a zone of inhibition with a diameter ≥ 2 mm formed by the combined application of the extract plus the antibiotic was observed, compared to the zone of inhibition formed by the action of the antibiotic alone. Antagonistic effect was considered when inhibition zones of smaller diameter were observed around the disks with antibiotic and extract, compared to that developed by the antibiotic alone. Indifferent effect was considered when there was a zone of inhibition surrounding the disks with antibiotic and extract, with a diameter equal to that resulting from the application of the antibiotic alone[24]. All activities were performed in triplicate.
3. Results and Discussion
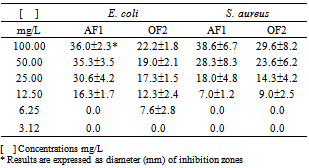
- The results shown in Table 1 were obtained by evaluation of the antimicrobial susceptibility and MIC determination in a Mueller-Hinton agar medium (MH-Merck) in different AF1 and OF2 concentrations and measurement of inhibition zone formed.In analyzing the data contained in Table 1, it appears that the MIC of AF1 for E. coli was 12.50 mg/L, whereas the MIC of OF2 was 6.25 mg/L. The MIC of both fractions for S. aureus was 12.50 mg/L.The MIC values obtained served as a base to evaluate the effect of these extracts on a group of antibiotics used in clinical therapy. Thus, a MIC equal to 12.50 mg/L was used as a standard in the interference tests of the metabolites contained in the fractions AF1 and OF2, when associated to molecules of antibiotics used in clinical therapy.
|
|
|
4. Conclusions
- This study shows that the oily (OF2) and aqueous (AF1) fractions from extracts of B. coridifolia can interfere on the activity of the antibiotics tested. Therefore, it is confirmed that the use of products of plant origin interfere with the effectiveness of some antibiotics during clinical therapy.
ACKNOWLEDGMENTS
- We would like to thank IBAMA - Brazilian Institute for the Environment, for the license for collection of biological material and the União de Ensino do Sudoeste do Paraná, for financing this project.
Conflict of Interest
- The authors declare no commercial or financial conflict of interest.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML