Liu Kedian
New Tech Institute, China
Correspondence to: Liu Kedian , New Tech Institute, China.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
This paper aims to recommend a novel atom model which can solve the problem that the prevailing physical atom model cannot be compatible with chemical atom model, especially the problem that prevailing physical atom model cannot explain chemical bonds. The novel atom model can not only explain almost all the physical phenomena that Bohr’s model cannot, but also can explain chemical bonds perfectly. Therefore it corroborates that the novel atom model is the only unique atom model of physics and chemistry.
Keywords:
Chemical bond, Covalent bond, Double bond, Triple bond, Coulomb force, Work function, Energy level transition, Atom model, Bohr’s model
Cite this paper: Liu Kedian , The Novel Atom Model that can Explain Chemical Bonds and Physical Phenomena is the Unique Atom Model of Physics and Chemistry, Physical Chemistry, Vol. 13 No. 2, 2024, pp. 32-39. doi: 10.5923/j.pc.20241302.02.
1. Introduction
Physicists or mathematicians want to give a thorough explanation by starting off a Hamiltonian operator of the system, whereas chemists want to give an accurate reduced depiction of analyzed phenomena by using drawings, schemes or pictures. However, mathematical expressions of physics and pictures of chemistry are always not unique or cannot explain each other.Prevalent attempts to reduce chemical explanations to physics are semi-successful or unsuccessful, vice versa prevailing physics laws always cannot explain chemical phenomena or drawings, properly. It is inescapable responsibility for physicists to apply physical models and laws to explain chemistry phenomena and improve drawings, schemes, depictions and diagrams of chemistry. This work can test and corroborate the rationality of physical and chemical atom models, it can also allow physical and chemical models to verify, modify, improve and correct their own assertions each other, thereby evolving to a more rational unified atom model.
2. Reasons for the Failure of Bohr Atom Model and Other Assertions
The main reasons for the failure of Bohr’s atom model are as following:1 Assume electrons are restricted to specific orbits, Bohr’s model does not explain why atoms absorb and emit light in such a way that can be easily determined by ear or eye.2 Bohr's model assumed that electrons moved in circular orbits at specific energy levels but do not emit radiation while in these stable orbits when they accelerate.3 It was unable to explain the Zeeman effects which occur when a spectral line is fragmented into many components in the presence of a magnetic field.4 It was unable to explain the Stark effect, which occurs when a spectral line is broken into fine lines in the presence of an electric field.5 It does not accurately describe the energy of atomic transition. Bohr's model described transitions between energy levels as instantaneous quantum jumps. But in fact, the energy level transition is a continuous process.6 Bohr's model was inadequate for explaining the electronic structure of atoms with more than one electron. Heavier elements presented far more complex electronic configurations, which required a more sophisticated approach.7 Bohr's model introduced several ad hoc assumptions, such as fixed orbits and the quantization of energy levels. These assumptions lack further theoretical explanation and divorces from physical realities.8 Bohr's model also cannot explain Compton effects.9 The most important, Bohr model is absent of explanation for chemical bonding. The Bohr model cannot provide explanation of chemical bonds and the sharing or transfer of electrons between atoms, which is basis of formation of molecules.The problems 1- 8 above can be explained by the novel physical atom model, but the details of the explanations are beyond the scope of this article and will just list out here not be discussed.In this paper, we will focus on the last problem (9), to explain the chemical bonds by the novel atomic model in detail.By the way, the electron cloudy assertion explains nothing at all!
3. The Novel Atom Model can Explain Most Problems that Led Bohr Model Failure
3.1. Briefing the Structure of the Novel Atom Model [3] [5]
In the novel atom model, the nucleus-electrons system is in a static equilibrium state between a universal static thermal repulsive conservative force and Coulomb force. The sum of repulsive forces and Coulomb forces is distributed in standing wave type in radial direction of spatial spherical surface layers (like layers of onion).The values of force on the spherical surface layer are equal.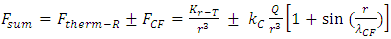 The “wavelength”
The “wavelength” 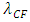 of the standing wave type distributed force field (thickness of each layer) is inversely proportional to the electric charge magnitude or quantity of Q:
of the standing wave type distributed force field (thickness of each layer) is inversely proportional to the electric charge magnitude or quantity of Q: 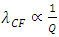 ,
, 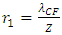 and
and 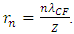 Therefore the total energy change (transition of energy levels) should be the same format with the prevailing equation
Therefore the total energy change (transition of energy levels) should be the same format with the prevailing equation 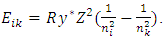 Single atom is being in a quasi crystal structure composed of nucleus-electrons, for example the metal of Beryllium before form crystal, its nucleus and electrons could be being in equilibrium state of attractive forces and repulsive forces as shown in Fig.3.1.
Single atom is being in a quasi crystal structure composed of nucleus-electrons, for example the metal of Beryllium before form crystal, its nucleus and electrons could be being in equilibrium state of attractive forces and repulsive forces as shown in Fig.3.1.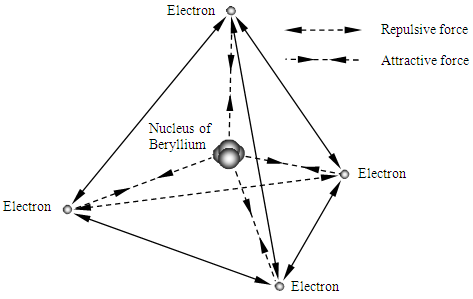 | Figure 3.1. Forces equilibrium state of bonds of quasi crystal structure of Beryllium |
The depiction of forces equilibrium of chemical bonds in molecules by novel atom model will be more detailed in section 5.1 of this paper.
3.2. Supplement and Improvement
Since any detection tools or methods for measurement of atomic structure have destructive action to the stationary states or locations of electrons, or will reconstruct or destroy the original structure of the atom. Thus Heisenberg’s uncertainty principle implies that the acquired phenomena of measuring are phenomena of destructed or destructing states or locations of electrons.Therefore, no doubt, the distribution of the force field layers and distribution of electrons should be more sophisticate than the preliminarily defined novel model in [3] [5].The distribution or redistribution of each electron affects the thickness (wavelength) of the sphere and the thickness of the next layer.The thickness (wavelength) of each shell layer is inversely proportional to the sum of charges contained in the shell.The thickness (wavelength) of each spherical shell is inversely proportional to the sum of charges it contains. Therefore the thickness of the layers of the “onion” could be increasing outward radially, as shown in Fig.3.2. 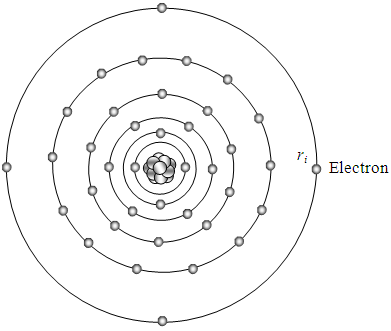 | Figure 3.2. The thickness (“wavelength”) of the layers of the “onion” could be increasing outward radially |
Of course, the novel atom model needs to be continuously improved, however the discovery of the novel atom model could reveal the concealed the key of more perfect atom model.
3.3. Briefing the Explanatory Power of the Novel atom Model [3] [5]
3.3.1. The Photoelectric Effect Actually is the Forward Energy Level Transitions of Electrons at Extreme Condition
The prevailingly so called work function Wa, is actually the work done by electron absorbed EM wave energy to overcome the attractive force of the first half cycle of the fluctuate force fields, 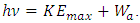 Whenever the critical frequency
Whenever the critical frequency 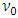 determined quanta energy is larger than hν, the energy of electron cancel firstly the negative force half cycle and continuously overcome the following fluctuated positive and negative forces to escape from the bind of the atom, as shown in Fig.3.3.1.
determined quanta energy is larger than hν, the energy of electron cancel firstly the negative force half cycle and continuously overcome the following fluctuated positive and negative forces to escape from the bind of the atom, as shown in Fig.3.3.1.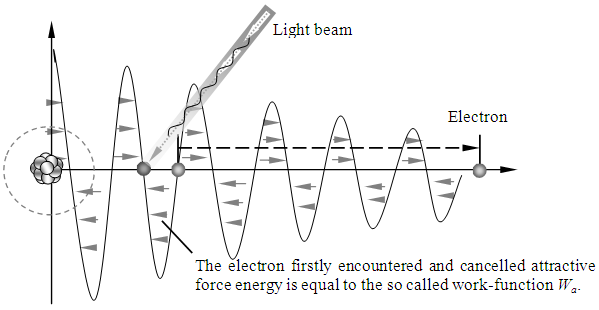 | Figure 3.3.1 |
3.3.2. The Actual Movement of Electrons Inside Atom to Generate EM Wave (Light Wave) Radiations
The electrons are stayed in the repulsive force and attractive force equilibrium positions of the force field spatial surface. When the electron moves backward a bit, the repulsive force will exert on it to repel it forward to equilibrium position. When the electron moves forward a bit, the attractive force will exert on it to draw it backward to equilibrium position. The electron energy levels transitions must continuously travel back and forth multiple cycles thus generate same frequency ν continuously then it is measurable. The electron travel back and forth multiple times (e.g., N times), every time it accelerates and decelerates (nk- ni) cycles thus emit N(nk - ni) cycles of EM wave, as shown in Fig.3.3.2.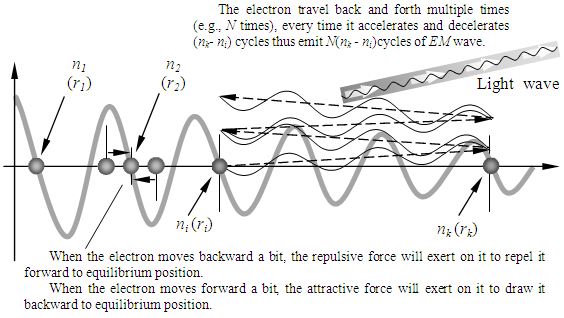 | Figure 3.3.2 |
3.4. Description of the Energy Levels of the Novel Atom Model
E.g., for i to i+4, the 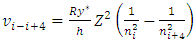 can be re-expressed as
can be re-expressed as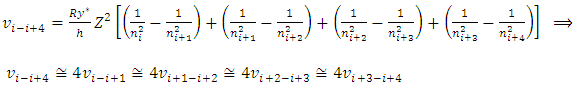 Since frequency is
Since frequency is 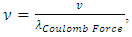 then the equations of the frequencies are
then the equations of the frequencies are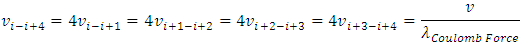 The electron will emit (k - i) cycles of same frequency
The electron will emit (k - i) cycles of same frequency 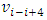 EM wave radiation during the transient of energy levels transition of level ni to level nk.
EM wave radiation during the transient of energy levels transition of level ni to level nk.
4. Explanation and Possible Depiction of Covalent Bonding by the Novel Atom Model (Possible Depiction of Covalent Bonding of NaCl for Example)
The possible depiction of covalent bonding of NaCl by novel atom model is shown as Fig.4.1.The so called chemical bond is actually working as a lock of forces equilibrium. Due to the actions of standing wave like distribution of the attractive force of bonding electron and repulsive force of nucleus, the system is locked in stable equilibrium of forces within a moderate range of distance variation.Only the same kind of force interacts. The thermal repulsive force is interacting on thermal repulsive force forever. We discuss only the interactions between positive-positive, positive-negative Coulomb forces. When the covalent electrons are bonding the nucleus, they are being in a stable equilibrium state of forces, as shown in Fig.4.1. Since there is only one interaction of repulsive force between the two nucleuses whilst there are two interactions of attractive force between the two nucleus and two electrons, therefore there is necessary condition of bonding.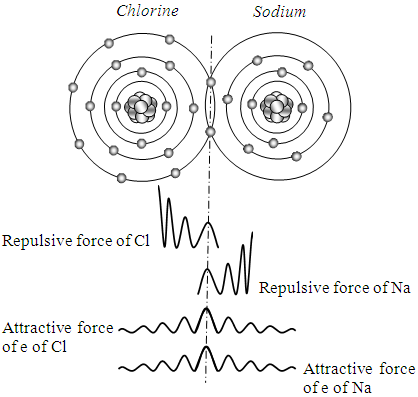 | Figure 4.1 |
When the two nucleus move away a bit as shown in Fig.4.2, the two repulsive force peaks of nucleus are not overlapping, the repulsive force is decreased whilst the positive peaks fall in the secondary peak of negative force peaks of electrons, therefore the attractive force will larger than repulsive force, the nucleus will be attracted backward to equilibrium position to be locked again.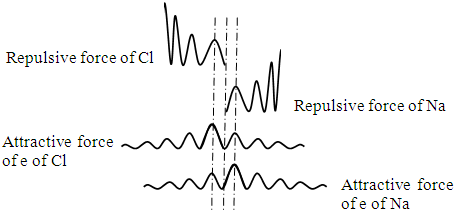 | Figure 4.2 |
When the two nucleus move nearer a bit as shown in Fig.4.3, the overlapping repulsive force peaks of nucleus are higher and higher, the repulsive force is increased sharply whilst the negative force peaks of electrons fall in the lower and lower positive force peaks of nucleus, therefore the repulsive force will larger than the attractive force, the nucleus will be repelled backward to equilibrium position to be locked again.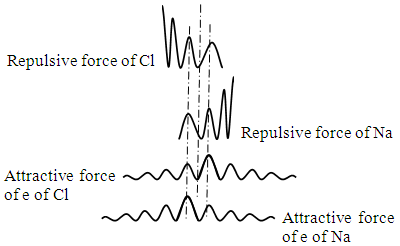 | Figure 4.3 |
5. Novel Atom Model Explains and Depicts Covalent Bonding of Typical Molecules
5.1. General Descriptions of Forces Equilibrium
The atoms that participate in covalent bonding share electrons in a way that enables them to acquire a static stable equilibrium of attractive forces and repulsive forces between involved electrons and nucleus. Actually they are being in a special crystal structure or a special monomer crystal structure. Like charges repel, while opposite charges attract. This is due to the nature of the electric force, which is a force that is exerted between two particles that have either opposite charges or similar charges. Molecules are being in a quasi crystal structure composed of atoms-electrons (chemical bonds)-atoms, as shown in Fig.5.1.1 and Fig.5.1.2.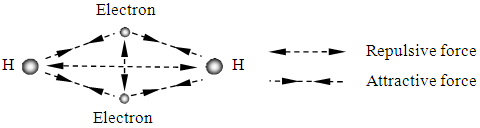 | Figure 5.1.1. Forces equilibrium state of bonds of quasi crystal structure of H2 |
 | Figure 5.1.2. Forces equilibrium state of bonds of quasi crystal structure of CH4 |
5.2. Descriptions of Covalent Bonds of Typical Species
5.2.1. Possible Depiction of Bonds of H2
Fig.5.2.1 shows the electrons share in the Hydrogen molecule. Explained by the novel atom model, the nucleus (protons) of hydrogen and electrons are in the equilibrium position (the circular) of attractive forces and repulsive forces of the particles. Two hydrogen atoms form a covalent bond to make a hydrogen molecule. Each contributes one electron and the electrons locate in the intersection points of the force fields spherical surfaces of equal value of repulsive and attractive forces. 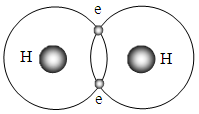 | Figure 5.2.1. Possible depiction of bonds of H2 |
(The depiction of equilibrium of the forces of the chemical bonds of the two atoms is similar to Fig.4.1. We do not repeat it again in the following sections.)
5.2.2. Possible Depictions of Bonds of Hydrogen Fluoride HF and Water H2O
We see that the water molecule contains two pairs of bonding electrons as shown in Fig.5.2.2 and hydrogen fluoride contains one pair shown in Fig.5.2.3.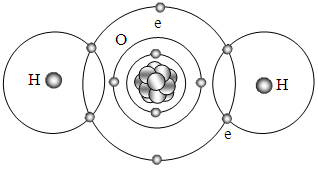 | Figure 5.2.2. Possible depiction of bonds of H2O |
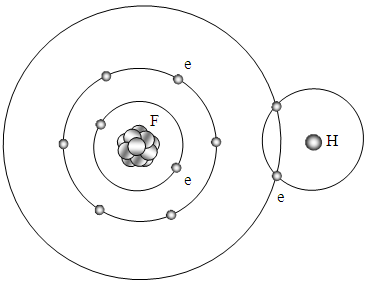 | Figure 5.2.3. Possible depiction of bonds of HF |
Similar to the structures of hydrogen molecules, the formation of stable hydrogen compounds for the next two nonmetals are oxygen and fluorine. Since the force field distributes in layers radially, the value of the forces are equivalent in a certain radius sphere surface. Therefore in a force field sphere surface, the electrons act as locks. The circulars represent equal value sphere surface of force field of a certain radius.
5.2.3. Possible Depiction of Bonds of Methane CH4
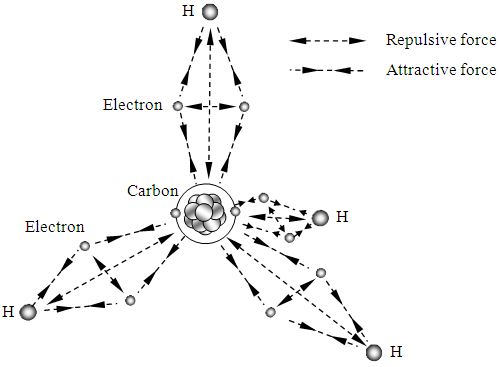
When electron share in the methane (CH4) molecule, 4 electrons in the valence shell of carbon combines with four hydrogen atoms to form a stable covalent compound where 8 electrons act as 8 locks to bond one carbon and four hydrogen to form CH4 molecule.The principle of bonds to form CH4 molecule is same as H2, we do not repeat. The circulars represent sphere surface of force field of equilibrium position, as shown in Fig.5.2.4.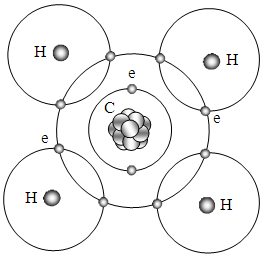 | Figure 5.2.4. Possible depiction of bonds of CH4 |
5.2.4. Possible Depiction of Bonds of Ammonia NH3 and BH3
The principle of bonds to form NH3 and BH3 molecules are same as CH4 and H2. As shown in Fig.5.2.5 (a) and Fig.5.2.5 (b).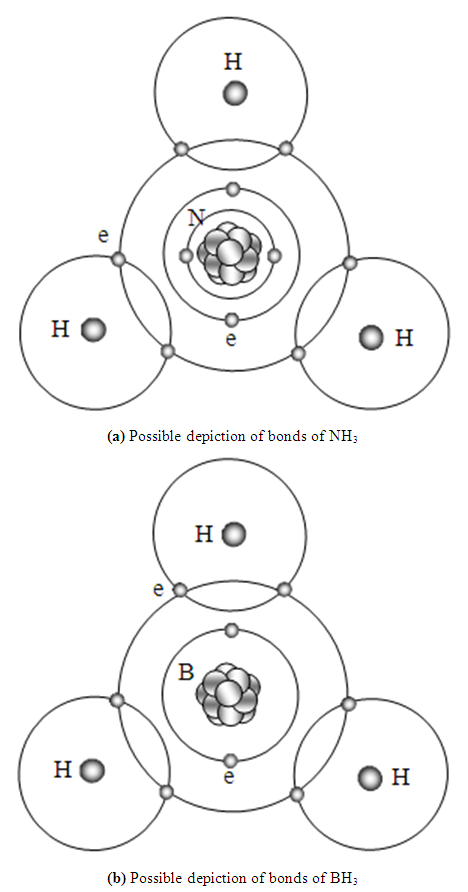 | Figure 5.2.5. Possible depiction of bonds of NH3 and BH3 |
5.3. Descriptions of Double Bond and Triple Bond of Typical Species
It is possible for two atoms bonded together to share 4 electrons. This bonding pattern is called a double bond. The ethylene molecule is an example as shown in Fig.5.3.1. Sharing of 6 electrons between two atoms is called a triple bond. The acetylene molecule provides an example of a triple bond as shown in Fig.5.3.2.
5.3.1. The Double Bond in Ethylene C2H4
Since the values of force on the spherical surface layer are equal, the 4 bonding electrons of the double bond are located in the intersection points of the two spherical force field surfaces of carbon to act as lock (bond).Every pair of electron shared by hydrogen and carbon also located in the intersection points of the two spherical force field surfaces of carbon and hydrogen to act as locks (bonds), as shown in Fig.5.3.1. The circulars represent the spherical equal force field surfaces.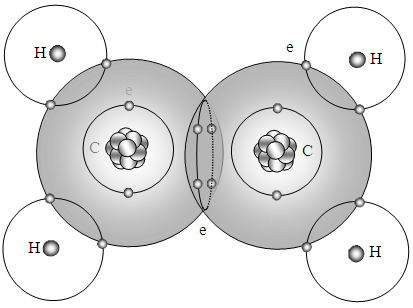 | Figure 5.3.1. Double bond of C2H4 |
5.3.2. The Triple Bond in Acetylene C2H2
Similar to the depiction of the double bond in ethylene C2H4, the 6 bonding electrons of the triple bond are located in the intersection points of the two spherical force field surfaces of carbon to act as lock (bond). Every pair of electron shared by hydrogen and carbon also located in the intersection points of the two spherical force field surfaces of carbon and hydrogen to act as locks (bonds), as shown in Fig.5.3.2. 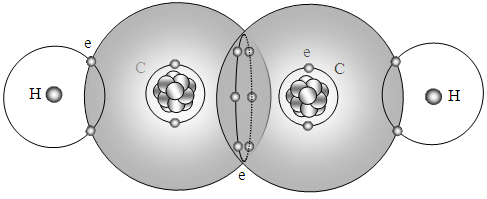 | Figure 5.3.2. Triple bond of C2H2 |
5.4. It is Worth Noting that
An atom in a polymeric organic molecule may share multiple bonds with other atoms with different force field sphere surface layers, and the neighbor bonds of an atom in a crystal structure shared with farer and nearer adjacent atoms should be different adequate force field sphere surface layers. (We do not discuss more details in this paper.)
6. Expectation of Further Researches
Honestly say, the novel atom model is a hypothetical judgment, one precondition (hypothesis) to support the model is the standing wave like distribution of the Coulomb force field. If this hypothesis is verified truth then the novel atom model will be completely verified and corroborated theoretically and practically and the model will be just perfect unique model of both chemistry and physics. Whatever, the hypothesis has great possibilities.Actually, the explanations of physical phenomena that Bohr’s model cannot explain and the explanation of chemical bond by the novel atom model is the direct verification and corroboration of the novel atom model; and the indirect verification of the standing wave like distribution of the Coulomb force of charged particles.Anyhow, we expect the direct verification of the standing wave like distribution finally.Generally, magnetic field is generated and accompanied by electric current. When charged particle is moving in rotational motion then generate magnetic field with magnetic field line of force. Accordingly we may inference that the static electric field could generate “static” magnetic field and the “static” magnetic field has no directional and has no magnetic field line of force. Therefore static electric field of charged particle could be a standing wave like layer distribution with sandwiched between each layer is “static” magnetic field layers.The verification of the hypothesis depends upon further researches and open minds of mainstream physics community.
7. Conclusions
The explanation of chemical bonds by the novel atom model together with the explanations of the physical phenomena that Bohr’s model and other model cannot explain verify and corroborate that the novel atom model can overwhelmingly replace Bohr’s atom model and other prevailing atom model. Therefore it verifies and corroborates that the novel atom model is the most reasonable unique atom model of physics and chemistry.
References
| [1] | Wolfgang Demtröder: Atoms, Molecules and Photons. Springer Oct 2010. |
| [2] | Liu Ke Dian: Discovery of a Universal Static Thermal Repulsive Force. American Academic Press 2022. |
| [3] | Liu Ke Dian: Correction of Fundamental Mistakes of Quantum Mechanics. American Academic Press 2023. |
| [4] | Liu Ke Dian: Discovery of a Universal Static Thermal Repulsive Force. International Journal of Theoretical and Mathematical Physics 2023, 13(3): 59-74 Scientific and Academic Publishing. |
| [5] | Liu Ke Dian: Derivation of a Most Reasonable Novel Atom Model. Physical Chemistry 2023, 12(1): 1-12 Scientific and Academic Publishing. |


 The “wavelength”
The “wavelength” 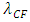 of the standing wave type distributed force field (thickness of each layer) is inversely proportional to the electric charge magnitude or quantity of Q:
of the standing wave type distributed force field (thickness of each layer) is inversely proportional to the electric charge magnitude or quantity of Q: 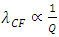 ,
, 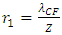 and
and 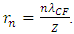 Therefore the total energy change (transition of energy levels) should be the same format with the prevailing equation
Therefore the total energy change (transition of energy levels) should be the same format with the prevailing equation 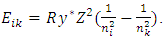 Single atom is being in a quasi crystal structure composed of nucleus-electrons, for example the metal of Beryllium before form crystal, its nucleus and electrons could be being in equilibrium state of attractive forces and repulsive forces as shown in Fig.3.1.
Single atom is being in a quasi crystal structure composed of nucleus-electrons, for example the metal of Beryllium before form crystal, its nucleus and electrons could be being in equilibrium state of attractive forces and repulsive forces as shown in Fig.3.1.

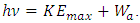 Whenever the critical frequency
Whenever the critical frequency 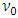 determined quanta energy is larger than hν, the energy of electron cancel firstly the negative force half cycle and continuously overcome the following fluctuated positive and negative forces to escape from the bind of the atom, as shown in Fig.3.3.1.
determined quanta energy is larger than hν, the energy of electron cancel firstly the negative force half cycle and continuously overcome the following fluctuated positive and negative forces to escape from the bind of the atom, as shown in Fig.3.3.1.

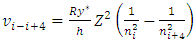 can be re-expressed as
can be re-expressed as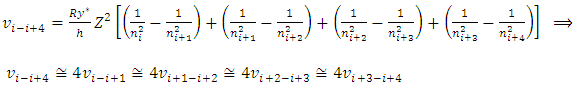 Since frequency is
Since frequency is 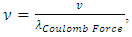 then the equations of the frequencies are
then the equations of the frequencies are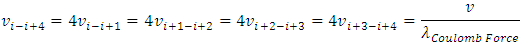 The electron will emit (k - i) cycles of same frequency
The electron will emit (k - i) cycles of same frequency 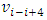 EM wave radiation during the transient of energy levels transition of level ni to level nk.
EM wave radiation during the transient of energy levels transition of level ni to level nk.











 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML