-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Physical Chemistry
p-ISSN: 2167-7042 e-ISSN: 2167-7069
2024; 13(2): 19-31
doi:10.5923/j.pc.20241302.01
Received: Mar. 2, 2024; Accepted: Mar. 12, 2024; Published: Mar. 29, 2024

Kinetics, Thermodynamic and Isotherms Modeling of the Equilibrium Sorption of Pb (II), Ni (II), and Cd (II) Ions into Tiger Nut Chaff (Cyperus Esculentus) from Model Wastewater
Olowu Rasaq Adewale1, Osundiya Medina Olubunmi1, Sobola Abdullahi Owolabi1, Osifeko Olawale Lawrence1, Tovide Oluwakemi Omotunde1, Oyewole Toyib Seun1, Elesho Adeseye Omololu1, Onifade Olayinka Omoniyi2, Majolagbe Abdulrafiu Olaiwola1, Onwordi Chionyedua Theresa1, Adejare Adeniyi Ayemu1
1Chemistry Department, Lagos State University, LASU Ojo
2Biochemistry Department, College of Medicine University of Lagos CMUL Idi-araba
Correspondence to: Olowu Rasaq Adewale, Chemistry Department, Lagos State University, LASU Ojo.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

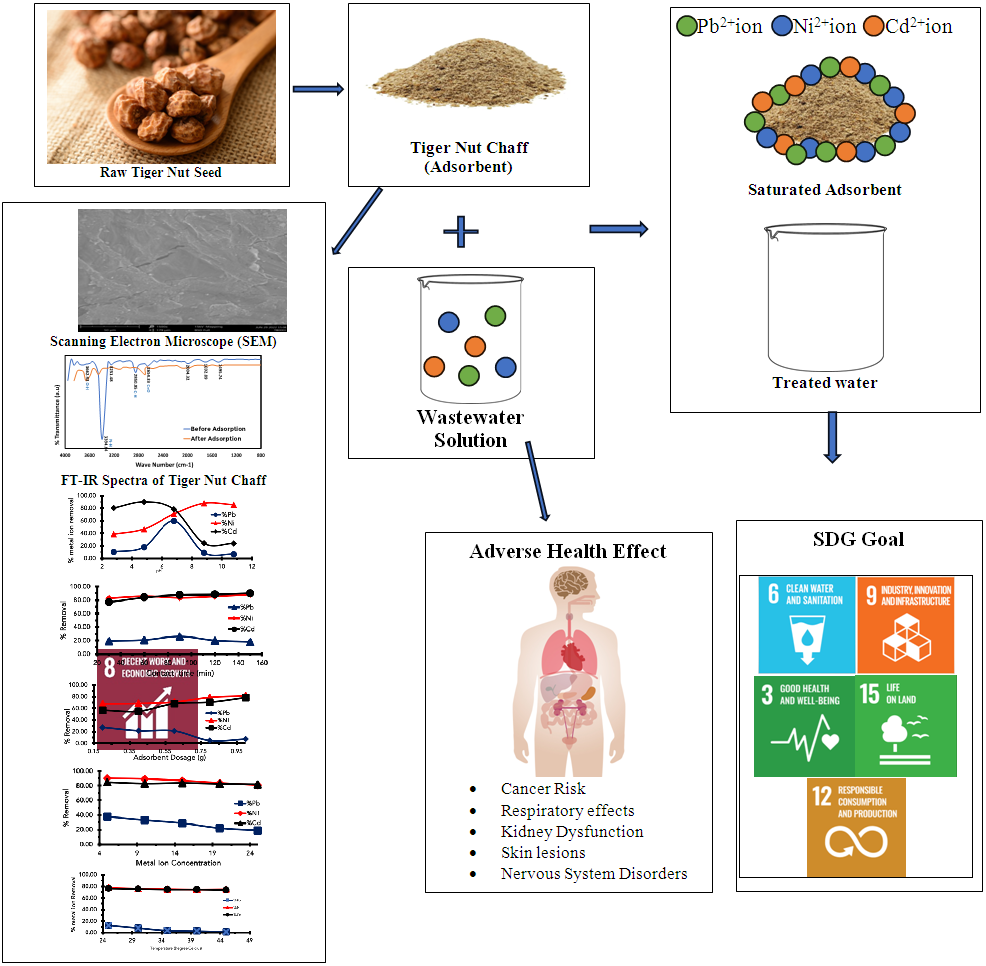
Heavy metals have detrimental effects on human health, and exposure to these metals has increased by industrial and anthropogenic activities and modern industrialization. The kinetics, isotherms, and thermodynamics of adsorption were investigated to establish the mechanisms of adsorption of tiger nut chaff as an alternative agricultural waste adsorbent in wastewater treatment applications. The sorption characteristics of the adsorbent was investigated under various experimental conditions, such as pH, contact time, initial concentration dose of Pb2+, Ni2+ and Cd2+ and sorbent mass. The thermodynamic studies showed that the adsorption process was feasible, exothermic and proceeded spontaneously, demonstrating that Tigernut (TN) chaff can be used as a sustainable alternative for the effective removal of Pb (II), Ni (II), and Cd (II) from model wastewater. Morphological characterization of the adsorbent before and after was carried out with the aid of a Scan Electron Microscopy (SEM) which revealed the adsorption of heavy metals on Tiger nut (TN) chaff. The FT-IR analysis revealed that likely functional groups responsible for the adsorption are C=O, NH and OH respectively. Adsorption of metal ion were pH dependent and the results indicate that the optimum pH for the removal of Pb (II), Ni (II), and Cd (II) ions was found to be 6.68 and the maximum percentage removal of Pb (II), Ni (II), and Cd(II) ionsat this pH were 59.80%, 70.75% and 78.48% respectively. Langmuir, Freundlich and Temkin adsorption Isotherm Models were used to simulate the equilibrium data. The experimental data were best fitted to Langmuir Isotherm model when compared with Freundlich and Temkin Isotherm model with the highest R2 values of 0.97, 0.99 and 0.99 for Pb (II), Ni (II), and Cd (II) ion respectively. The study showed that the pseudo second-order kinetic model had the best correlation for all the adsorption kinetic experimental data for the prepared adsorbent inferring that the rate-controlling step during the Pb (II), Ni (II), and Cd (II) ions adsorption onto the prepared adsorbents is chemisorption and this revealed that the biomass of tiger nut (TN) is a promising cost-effective biosorbent for heavy metal removal from the environment.
Keywords: Cyperus esculentus, Bioaccumulation, Heavy Metals, Kinetics, Thermodynamics, Biosorption
Cite this paper: Olowu Rasaq Adewale, Osundiya Medina Olubunmi, Sobola Abdullahi Owolabi, Osifeko Olawale Lawrence, Tovide Oluwakemi Omotunde, Oyewole Toyib Seun, Elesho Adeseye Omololu, Onifade Olayinka Omoniyi, Majolagbe Abdulrafiu Olaiwola, Onwordi Chionyedua Theresa, Adejare Adeniyi Ayemu, Kinetics, Thermodynamic and Isotherms Modeling of the Equilibrium Sorption of Pb (II), Ni (II), and Cd (II) Ions into Tiger Nut Chaff (Cyperus Esculentus) from Model Wastewater, Physical Chemistry, Vol. 13 No. 2, 2024, pp. 19-31. doi: 10.5923/j.pc.20241302.01.
Article Outline
1. Introduction
- Heavy metals have harmful effects on human health, and exposure to these metals has been on the increase and contamination of water by toxic metals is an environmental concern and hundreds of millions of people are being affected around the world [1]. The discharge of these pollutants into the environment, especially water bodies has been on the rising due to urbanization and industrialization such as pharmaceutical, textile, paints production, metal finishing, mining, battery manufacturing and electroplating respectively [1-2]. Huge number of waste produce from these industries contains heavy metals which is the cause of environmental pollution [2]. These heavy metals are toxic or carcinogenic, persistent, non-biodegradable, and present danger to human health therefore most of the waste water from industries such as metal plating, mining operations, tanneries and radiator manufacturing, smelting, alloy industries, textile industry, dye industries effluents need to be treated before been discharged into the aquatic environment [1-2]. Food contamination with heavy metals is another concern for human and animal health and the concentration of heavy metals in water resources, air, and food is assessed with this regard [3-4]. Several acute and chronic toxic effects of heavy metals affect different body organs. Gastrointestinal and kidney dysfunction, nervous system disorders, skin lesions, vascular damage, immune system dysfunction, birth defects, and cancer are examples of the complications of heavy metal toxicity [5-6]. Lead is a bluish-white lustrous metal. It is very soft, highly malleable, ductile, and a relatively poor conductor of electricity. It is very resistant to corrosion but tarnishes upon exposure to air [6]. Lead can end up in water and soils through corrosion of leaded pipelines in a water transporting system and through corrosion of leaded paints. It cannot be broken down and converted to other forms. Lead is a particularly dangerous chemical, as it can accumulate in individual organisms and also in entire food chains [6-8]. Nickel is silvery-white, hard, malleable, and ductile metal. It is of the iron group and it takes on a high polish. It is a fairly good conductor of heat and electricity. For animals, nickel is an essential foodstuff in small amounts. But nickel is not only favorable as an essential element; it can also be dangerous when the permissible amounts are exceeded and can cause various kinds of cancer on different sites within the bodies of animals [6,9]. Cadmium (Cd) is a soft, malleable, bluish white metal found in zinc ores, and to a much lesser extent, in the cadmium mineral greenockite. Most of the cadmium produced today is obtained from zinc by-products and recovered from spent nickel-cadmium batteries. Cadmium waste streams from the industries mainly end up in soils. The causes of these waste streams are for instance zinc production, phosphate ore implication and bio industrial manure. Cadmium waste streams may also enter the air through household waste combustion and burning of fossil fuels. Due to regulations only little cadmium now enters the water through disposal of wastewater from households or industries [6,10-13]. Simultaneous exposure to two or more metals may have cumulative effects. [14-16]. Tigernut (Cyperus esculentus), an underutilized crop, was found to be high in dietary fiber content, suggesting that it could be useful in the treatment and prevention of a variety of diseases, including colon cancer, coronary heart disease, obesity, diabetics, and gastro-intestinal disorders [17-19].There are various methods for heavy metal removal from aqueous effluents, such as electroplating, ion exchange, membrane separation, adsorption, etc. Among all the aforementioned techniques, adsorption is the most simple, efficient, and economically friendly. Adsorption emerged to be good for the treatment of effluents and the first thing for an efficient adsorption process is the search for a low cost adsorbent with high adsorption capacity and high biodegradability tendency [20-21].This study therefore establish the optimal conditions for the use of tiger nut chaff for the removal of heavy metals from model wastewater as a sustainable alternative agricultural waste adsorbent in wastewater treatment applications.
2. Materials and Methods
2.1. Preparation of Stock Solution
- A stock solution of 1000 mg/L of soluble salt of Pb (II), Ni (II), and Cd (II) was prepared dissolving their respective water-soluble salts of PbCl2, Ni(NO3)2 and CdCl2 in deionized water respectively; then the working solution was prepared from the stock. The pH of each of the working solutions was adjusted to a desired value by drop-wise addition of 0.1M HCl and NaOH.
2.2. Collection and Preparation of Adsorbent
- The Tiger nuts (TN) were procured from first Gate market, along Lasu-Iba road, Iba local Government of Lagos State. The TN was washed with tap water first, then distilled water for conspicuously to remove dirt, dust and other impurities. The juicy parts were extracted, and residual waste materials were then dried, pulverized and sieved then sun dried for several weeks. The powdered form of TN was stored and used for the study. No other physical or chemical treatment was employed prior to adsorption experiments.
|
2.3. Batch Adsorption Experiment Orbital Shakers Model SSM1, SSL1 - CCL
- The pH variation: The effect of pH on the equilibrium adsorption of metal ions by TN was investigated over a range of pH (2.68, 4.68, 6.68, 8.68, and 10.68) values at an initial concentration of 20 mg L-1. The suspension was agitated using an orbital shaker (Model SSMI, SSL1-CCL) at a speed of 250 rpm for 60 minutes at 25°C, left to settle, and then filtered.At an optimal pH of 6.68 and a temperature of 25°C, 100 ml of 20 mg L-1 metal ions solution was used with varying dosages (0.2, 0.4, 0.6, 0.8, and 1.0 g) of the adsorbent.The concentration variation: At optimal pH, temperature, and dosage of 0.6, 25°C and 1.0 g, 100 mL of the metal ions solution with varying concentrations (5, 10, 15, 20, and 25mgL-1) was used.Time variation: 100 mL of 20 mg/L of metal ion solution at pH 6.68 with 1.0 g of the adsorbent was used. The suspension was agitated using an orbital shaker at a speed of 250 rpm at time intervals of 30, 60, 90, 120, and 150 minutes respectively. After shaking, the suspension was left to settle and was filtered.Temperature variation: 100 mL of metal ion solution at an optimal pH of 6.68 with 1.0 g of the adsorbent was used. The mixture was agitated using an orbital shaker at a speed of 250 rpm at varying temperatures (25, 30, 35, 40, and 45°C) for 60 minutes.Data TreatmentCalculation of the removal of metal ions by tiger nuts adsorbentThe adsorption capacities were determined according to the following equation:
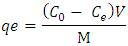 Where;
Where; 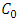 and
and 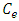 are the initial and final sorbate concentration in solutions,
are the initial and final sorbate concentration in solutions, 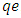 , V and M are the amount of sorbate sorbed (mg/g), volume of the solution (ml) and mass (g) of TN sample, respectively.Calculation of the percentage of metal ion removed by the adsorbent (TN).The percentage removal of metal ions was calculated using the following equation;
, V and M are the amount of sorbate sorbed (mg/g), volume of the solution (ml) and mass (g) of TN sample, respectively.Calculation of the percentage of metal ion removed by the adsorbent (TN).The percentage removal of metal ions was calculated using the following equation;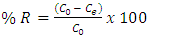 | (1) |
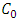 is the initial metal ions concentration in solution (mg/L);
is the initial metal ions concentration in solution (mg/L); 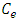 is the metal ions concentration removed by adsorbent at equilibrium (mg/L).
is the metal ions concentration removed by adsorbent at equilibrium (mg/L).2.4. Adsorption Isotherm
- The characteristics of adsorbates and the adsorbents were described by the Freundlich and Langmuir adsorption equilibrium equations. The Freundlich isotherm is used to describe adsorption processes that occur on heterogeneous surfaces and active sites with different energies based on multilayer adsorption and equilibrium [22]. The Freundlich equation can be expressed as;
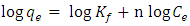 | (2) |
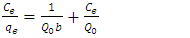 | (3) |
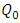 and b are Langmuir equilibrium coefficients,
and b are Langmuir equilibrium coefficients, 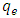 is the amount of metal ions adsorbed per unit mass of the adsorbent, Ce is the equilibrium solution concentration.
is the amount of metal ions adsorbed per unit mass of the adsorbent, Ce is the equilibrium solution concentration.2.5. Thermodynamics of Adsorption
- The thermodynamic parameters of Gibbs free energy
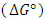 , enthalpy
, enthalpy 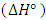 , and entropy
, and entropy 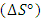 are used in the determination of spontaneity of the adsorption process, the nature of the adsorption process, and also the adsorbent applicability [24]. The parameters
are used in the determination of spontaneity of the adsorption process, the nature of the adsorption process, and also the adsorbent applicability [24]. The parameters 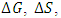 and
and 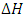 were evaluated using the expressions in Equation below;
were evaluated using the expressions in Equation below;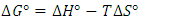 | (4) |
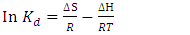 | (5) |
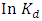 against 1/T.
against 1/T.  is the equilibrium constant.
is the equilibrium constant.2.6. Adsorption Kinetics Modeling
- Lagergren first order and second order equation are used in the determination of adsorption interactions. A study on adsorption of Pb2+, Ni2+ and Cd2+ from model waste water by Tiger Nut Chaff was done.Lagergren First Order Equation: The pseudo-first-order kinetic model explains the relationship between the rate the sorption sites of the adsorbents is occupied and the number of unoccupied sites. It is defined using the Lagergren equation [25] as follows:
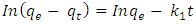 | (6) |
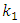 is the rate constant of the equation;
is the rate constant of the equation; 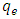 is the amount of adsorption equilibrium (mg/g). The adsorption rate constant
is the amount of adsorption equilibrium (mg/g). The adsorption rate constant 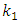 , can be determined experimentally by plotting of
, can be determined experimentally by plotting of 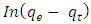 against t [25-26].Second Order Equation: The pseudo-second-order kinetic model describes the dependency of the adsorption capacity of the adsorbent on time and can be determined based on Equation (7) expressed as
against t [25-26].Second Order Equation: The pseudo-second-order kinetic model describes the dependency of the adsorption capacity of the adsorbent on time and can be determined based on Equation (7) expressed as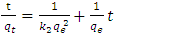 | (7) |
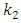 is the rate constant of the second-order-equation;
is the rate constant of the second-order-equation; 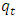 is the amount of adsorption time t (min) and
is the amount of adsorption time t (min) and 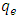 is the amount of adsorption equilibrium (mg/g) [22]. The linear plot of t/qt against time is used to determine qe and k2 from the slope and intercept, respectively.
is the amount of adsorption equilibrium (mg/g) [22]. The linear plot of t/qt against time is used to determine qe and k2 from the slope and intercept, respectively.3. Result and Discussion
3.1. Scanning Electron Microscope (SEM) Analysis
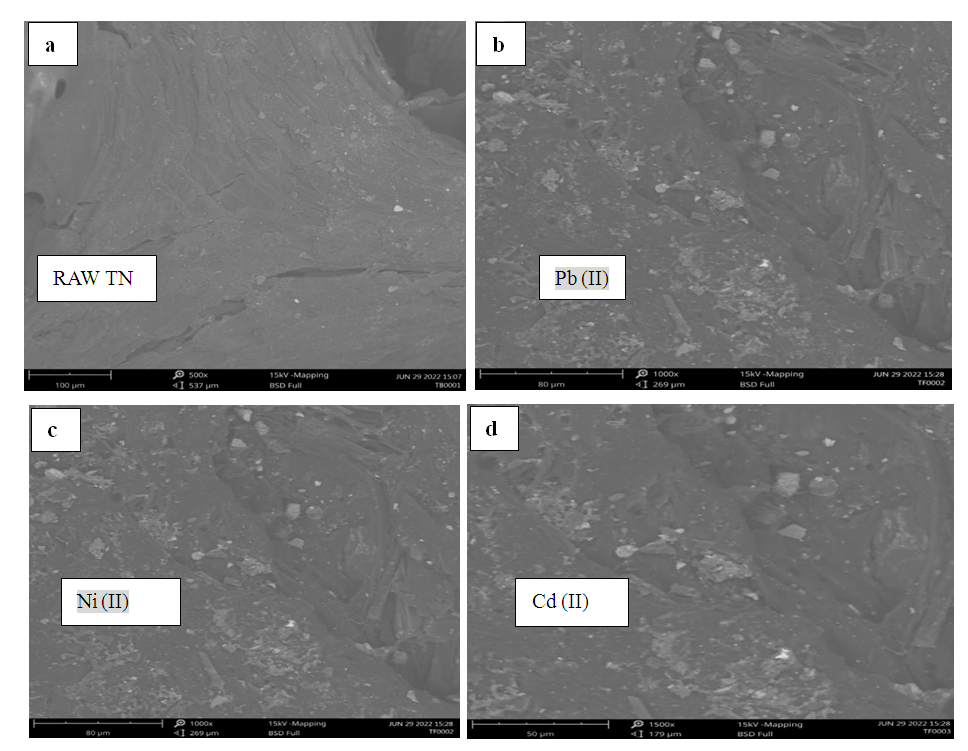
- Scanning Electron Microscopy (SEM) (aTecnai G2F2OX-TTwinMAT model) was utilized to investigate the morphological characteristics of biomass prior to and following adsorption studies. Raw tiger nut chaft exhibited a greater number of pores, which facilitates the adsorption process due to its surface reaction nature. The presence of pores is evident from Fig. 1a-d, which depicts a homogeneously distributed network of small filamentous and fistulous crystallites. The matrix also exhibits luminous and non-luminous features, indicating the presence of minerals dispersed throughout the organic matrix and on the surface. In addition, various features such as fissures, cleats, cracks, and veins are visible. The surface appears bright and mostly projected. The micrographs indicate the presence of numerous fragmented particles resembling spots, which are evident in the SEM images of raw tiger nut chaff before adsorption (Fig. 1a), showing the porous nature of the adsorbent suitable for target metal adsorption. Fig. 1b-d exhibit a rough and flaky surface compared to Figure 1a, suggesting that the pores present on raw tiger nut chaff before adsorption have been successfully occupied by metal ions after adsorption.
3.2. Fourier Transform Infrared Spectroscopic (FT-IR) Analysis
- Tiger nut biosorbent was analyzed using a NicoletTM iS50 FT-IR spectrophotometer to authenticate the possible interactions of heavy metal ions onto biosorbent and its effect on the biosorption behavior. The FT-IR spectra of the biomass (TN) showed several absorption bands between the wavelength ranges of 4000 - 400cm-1 showing transmittance of the biosorbent. Figure 2 showed the various peaks and identified functional group for tiger nut (TN), within the specified wavelength range of 4000 cm-1-1500 cm-1, the broad peak at 3662.92 cm-1 is an indication of the presence of vibrational stretching of the bonded –OH group [26]. The low-intensity bands observed at peaks 2850.05 cm-1 indicate the presence of the stretching C–H bond vibrations [26-27]. The peaks at 2654.33 cm-1 revealed the presence of C=O while the peaks at 1692.82 cm-1 indicated the presence of the C=N (imine or oxime) group. [26]. The peaks at 1456.30 cm-1 indicate the presence of aromatic C=C. Furthermore, the TN biosorbent, in the functional group region shows a weak intensity and shift of the peak at 3450.50 cm-1 from 3393.64cm-1 before adsorption which indicated the presence of an amine (N-H) group [28].
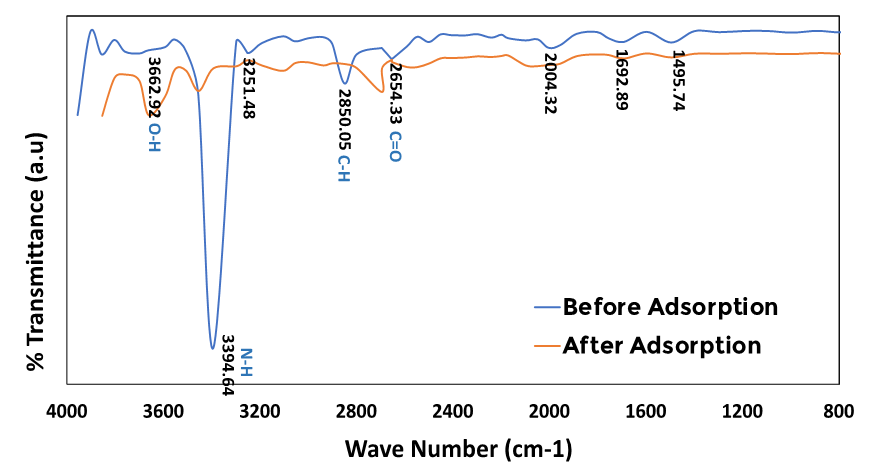 | Figure 2. FT-IR Spectra of Tiger Nut Chaff(Cyperus esculentus) before and after adsorption |
3.3. Effect of Parameters on Adsorption Studies
3.3.1. Effect of pH on Adsorption Studies
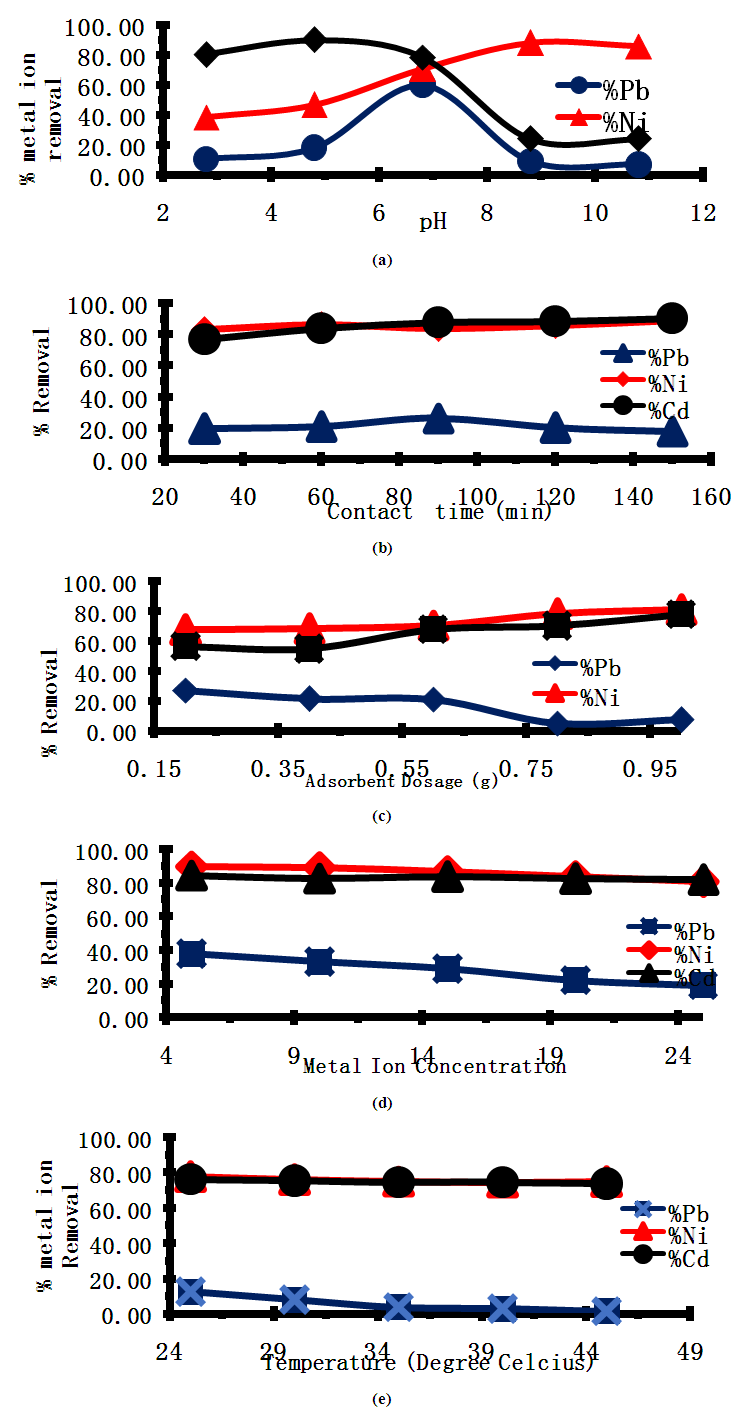
- The effects of pH on the adsorption of Pb (II), Ni (II), and Cd (II) ions were investigated by batch adsorption experiments at varying pH values of 2.68 4.68, 6.68, 8.68, and 10.68 with all other experimental variables kept constant. The effects of pH on Pb (II), Ni (II), and 4.68 Cd (II) metal ion adsorption onto Cyperus esculentus are illustrated in Figure 3a. It can be seen that the pH values had significant effects on the adsorption process. The percentage efficiency was better in the acidic and alkaline pH ranges. During this study, results revealed that the removal of metal ions was strongly dependent on the pH of the solution. The maximum removal capacity of Cyperus esculentus was found to be at pH 6.68 for Pb (II), 8.68 for Ni (II), and 4.68 for Cd (II). Percent metal ions removal increased rapidly with the increase in pH of the solution initially with further increase in pH causing a drastic decrease in the adsorption percentage. This might be due to the weakening of the electrostatic force of attraction between the oppositely charged adsorbate and adsorbent which ultimately leads to the reduction in sorption capacity [24].
3.3.2. Effect of Contact Time on Adsorption
- The effect of contact time on the adsorption of heavy metals on tiger nut chaff was investigated at constant initial metal ion concentration of 20 mg/l for Pb (II), Ni (II) and Cd (II) ions respectively at varying adsorption times from 30 - 150 min. Contact time is one of the most effective factors in the batch adsorption process. The result of the effect of contact time on adsorption as shown in figure 3b reveals that adsorption of Pb (II), Ni (II) and Cd (II) ions onto the tiger nut (TN) chaff adsorbent increased with an increase in contact time and was rapid. The percentage of Pb(II), Ni(II), and Cd(II) ions. ions adsorbed by TN was at optimum at 90 mins for Pb(II) and 150 mins for both Ni(II), and Cd(II) ion respectively.
3.3.3. Effect of Adsorbent Dosage on Adsorption Studies
- The effect of adsorbent dosage on the adsorption of Ni (II),Cd (II) and Pb (II) ions was studied at a constant initial metal ion concentration of 20 mg/l for Pb2+, Ni2+and Cd2+ ions at a constant pH of 6.68 and constant agitation time of 60 minutes. Figure 3c shows that the adsorption of Ni (II) and Cd (II) ions increased with an increase in dosage except for Pb (II) ion which exhibited a decrease with an increase in dosage. Ni (II), and Cd(II) ions increased from 67.70% to 81.16% and 56.57% to 78.16% with an increase in the adsorbent dose of 0.2g to 1g respectively while the percentage elimination of Pb (II) decreased from 27.24% to 5.02 % with increasing adsorbent dosage of 0.2g to 1g.
3.3.4. Effect of Initial Metal Ion Concentration on Adsorption Studies
- The percentage of Pb (II), Ni (II), and Cd (II), ions adsorbed by Tigernut TN decreases with an increase in the initial metal concentration. From the analysis obtained for metal ion concentration, it was observed that the adsorption efficiency decreases progressively with an increase in the initial metal concentration of Pb (II), Ni (II), and Cd (II), respectively as shown in figure 3d.
3.3.5. Effect of Temperature on Adsorption Studies
- The effect of temperature on the adsorption process was studied at varying temperatures from 25°C to 45°C as shown in Figure 3e. It was observed that the adsorption of metal ions decreases with the increase in temperature. This indicates that the adsorption process is exothermic. The decrease in Pb (II), Ni (II) and Cd (II), ion concentrations with increasing temperature indicates a low energy requirement for the metal ions onto the Tiger nut chaff. This decrease could be attributed to a decrease in ion mobility due to a decrease in the number of active surface sites for adsorption by tiger nut chaff at higher temperatures thus decreasing the number of ions that interact with active sites of the biosorbent [30].
3.4. Adsorption Isotherms Studies
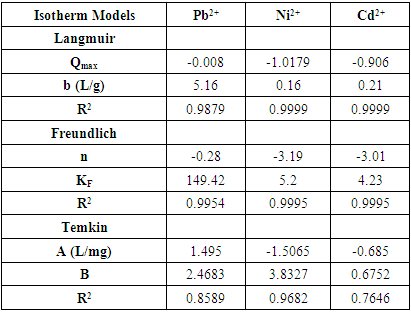
- The research examined the adsorption of lead, nickel, and cadmium ions onto tiger nut chaff using the Langmuir, Freundlich, and Temkin isotherm models. The results of the study, as presented in Table 2, indicate that the Langmuir and Freundlich models provide a good fit for the data, with R2 values ranging from 0.9954 to 0.9995 as shown in figures 4a-c. The correlation coefficient values suggest that the Langmuir model is a better fit for nickel and cadmium ions, while the Freundlich model is more appropriate for lead ions. The maximum adsorption capacity, qm, was calculated from the Langmuir plots for each metal ion. The analysis revealed that tiger nut chaff has a higher adsorption capacity for lead compared to nickel and cadmium. However, the values obtained in this study are relatively low compared to previously reported adsorption capacities [31]. Nonetheless, tiger nut chaff is an inexpensive and readily available food source that could potentially be utilized for metal ion removal from industrial wastewater. The Freundlich isotherm model was found to be appropriate for the adsorption of lead, nickel, and cadmium onto tiger nut chaff. The values of Kf and n were determined from the intercept and slope of the graph for each metal ion, respectively, and are also presented in Table 2. The results indicate that lead has the strongest adsorption tendency towards tiger nut chaff, followed by nickel and cadmium. Additionally, the n values obtained for the metal ions adsorption onto tiger nut chaff are less than unity, suggesting that the adsorption process is chemical in nature.
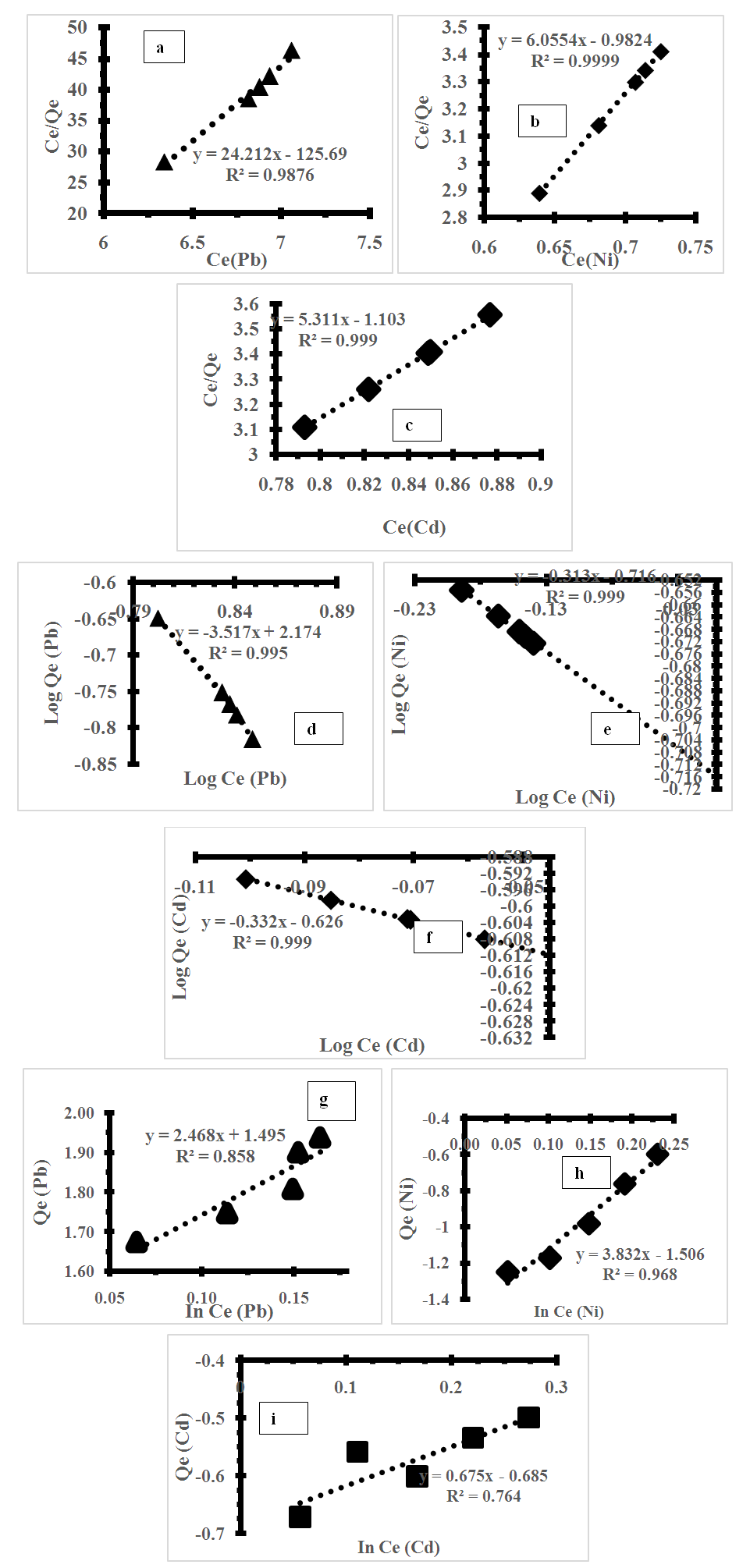 | Figure 4. Adsorption isotherm plot (a,b,c) Langmuir for Pb (II), Ni (II) Cd (II), (d,e,f) Freundlich for Pb (II), Ni (II) Cd (II) and (g,h,i) Temkin for Pb (II), Ni (II) Cd (II) |
|
3.5. Adsorption Kinetic Studies
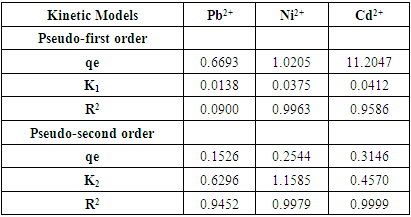
- To examine the impact of time on the adsorption process and determine the service time of the adsorbent, the adsorption kinetic of the metal ions using the prepared adsorbents was assessed. Elovich's pseudo-first and pseudo-second-order models were applied to analyze the experimental data and identify potential mechanisms involved in adsorption [1]. The adsorption kinetics are illustrated in Figure 5a-f and the model fitting parameters are summarized in Table 3. The results suggest that the experimental data was better fitted by the pseudo-second-order reaction than by the pseudo-first-order reaction.
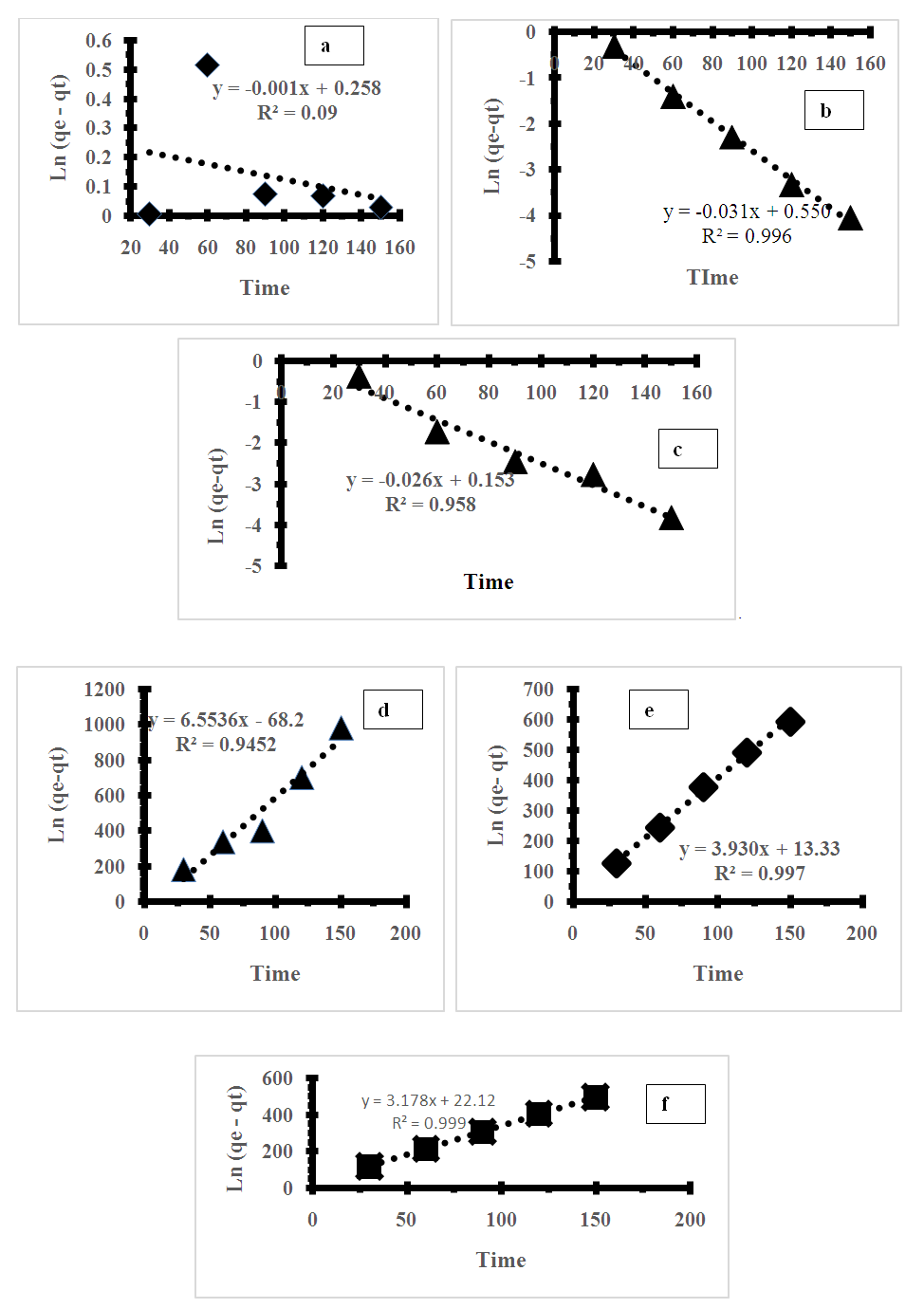 | Figure 5. Pseudo first order plot (a,b,c) for Pb (II), Ni (II) Cd (II), and Pseudo second order plot (d,e,f) for Pb (II), Ni (II) Cd (II) |
|
3.6. Thermodynamic Studies on Adsorption
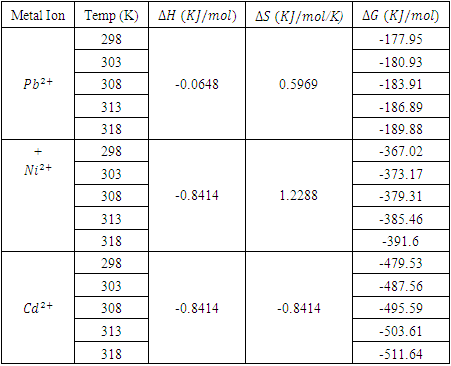
- The standard enthalpy ∆H° and entropy ∆S° were established from the slope and intercept of the graph of ln K against T-1, as depicted in Figure 6 and Table 4. A negative value was obtained for ∆G° across all temperatures investigated. This finding confirms the spontaneous nature of the adsorption process. The thermodynamic analysis demonstrated that the adsorption process was feasible, exothermic, and proceeded spontaneously. The overall outcomes illustrate the potential application of Cyperus esculentus as a sustainable adsorbent for the elimination of Pb (II), Ni (II), and Cd (II) ions from model wastewater.
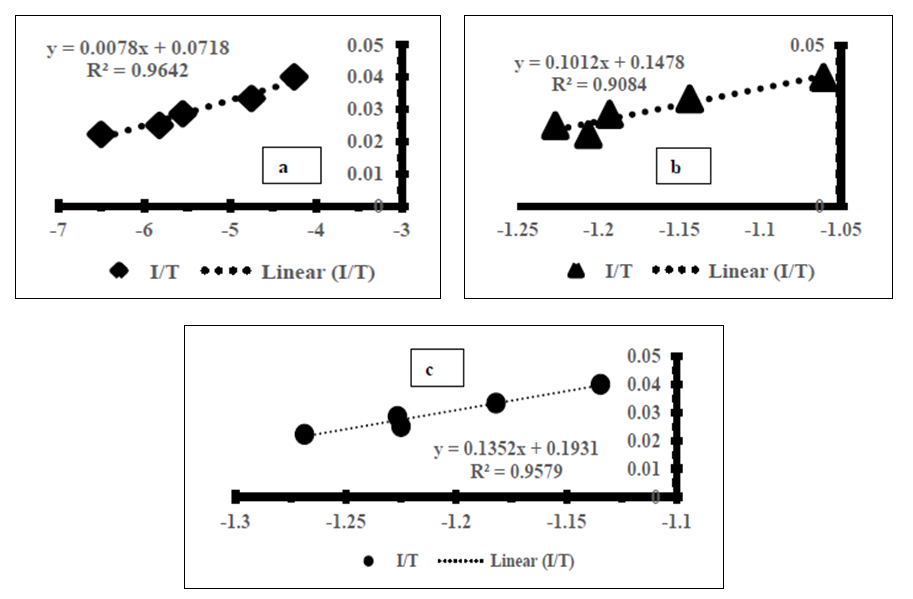 | Figure 6. Plot of Van’t Hoff for the adsorption of (a) Pb (II) (b) Ni (II) and (c) Cd (II) onto Tiger nut ((Cyperus esculentus) |
|
4. Conclusions
- Given the importance of efficient wastewater treatment and the need to safeguard human health by removing heavy metals from wastewater, tiger nut (TN) biomass has emerged as a promising biosorbent for the removal of Pb(II), Ni(II), and Cd(II) ions. This is due to its good adsorption capacity, ease of sample treatment, and availability. The biosorbent demonstrated an increased ability to remove these ions at both acidic and basic pH, with the optimal removal at a slightly acidic pH of 6.68. The pseudo-second-order kinetic model was a better fit for the adsorption system than the pseudo-first-order model, with a higher correlation coefficient. Thermodynamic studies revealed that the adsorption process was exothermic and spontaneous, with a negative Gibbs free energy of adsorption. The isotherm and thermodynamic data indicated that the adsorption mechanism was mainly chemisorption. This research work aligns with the UN Sustainable Development Goals by enhancing efficient wastewater treatment and safeguarding human health through the removal of heavy metals from wastewater.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML