Liu Kedian
China
Correspondence to: Liu Kedian, China.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
This paper aims to present the derivation of a most reasonable novel atom model. In the novel atom model, the nucleus-electrons system is in a static equilibrium state between a universal static thermal repulsive conservative force and Coulomb force.
Keywords:
Repulsive force, Coulomb force, Work function, Energy level, Atom model, Electrons, Nucleus, Bohr’s model
Cite this paper: Liu Kedian, Derivation of a Most Reasonable Novel Atom Model, Physical Chemistry, Vol. 12 No. 1, 2023, pp. 1-12. doi: 10.5923/j.pc.20231201.01.
1. Introduction
According to the equation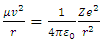 | (1.0) |
we see that the velocity v in Eq(0.1) that determine the centripetal force 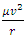 is only the tangential component (vx). The velocity vy of the vertical direction y is not taken into account. One single matter or electron has no conditions to form a standing wave or standing matter wave. There are no boundary conditions of standing wave for a single electron, let alone multiply electrons in same orbit. Therefore Bohr’s model is groundless.The electrons and the nucleus must be in relative static stable equilibrium state at different (potential) energy levels, rather than in kinetic energy levels.
is only the tangential component (vx). The velocity vy of the vertical direction y is not taken into account. One single matter or electron has no conditions to form a standing wave or standing matter wave. There are no boundary conditions of standing wave for a single electron, let alone multiply electrons in same orbit. Therefore Bohr’s model is groundless.The electrons and the nucleus must be in relative static stable equilibrium state at different (potential) energy levels, rather than in kinetic energy levels.
1.1. Briefing the Key Point of the Book Discovery of a Universal Static Thermal Repulsive Force and one Hypothesis about Coulomb Force
1.1.1. The Key Point of Universal Static Thermal Repulsive Force
The universal static thermal repulsive force between gas molecules is normally represented as [5] 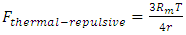 | (1.1) |
where T is absolute temperature, Rm is gas constant of one molecule; r is the radius distance of the force acting position to molecule center.The thermal repulsive force between two spheres is inversely proportional to 3th power of radius r, written as [5]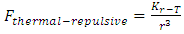 | (1.2) |
There could be a reasonable postulat that in the equilibrium position (or a spatial spherical surface) between (gravitational) attractive force and thermal repulsive force, the matters have only freedoms of motion thus can move only in the spatial spherical surface in tangential direction) [5]. The most typical examples of such equilibrium are the equilibrium of solar system and electrons-nucleus system; they are laid in equilibriums in between (gravitational) attractive force and thermal repulsive force.
1.1.2. A Hypothesis about Electric Field of Nucleus
The electric field of the nucleus is different from Gaussian’s description: instead, the electric field of the nucleus could be distributed in standing wave type in radial direction.
1.2. The Velocity Function of the Electron During the Energy Level Transition in Atom Must be Sinusoidal Form
1.2.1. Re-analyzing the Details of Energy Level Transition in Bohr’s Model
(Reviewing the inconsistent of Bohr’s model)1 The equation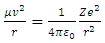 | (2.1) |
implies that the centripetal force is in the equilibrium with the Coulomb force, we assume it is being tenable.2 Without restoring conservative reversible force, the de Broglie matter wave condition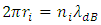 | (2.2) |
(where 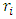 is radius of energy level i,
is radius of energy level i, 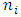 is quantum number of energy level i,
is quantum number of energy level i, 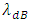 is de Broglie’s wavelength) is purely an imagination. It does not matter to temporarily believe it being true; however, the condition
is de Broglie’s wavelength) is purely an imagination. It does not matter to temporarily believe it being true; however, the condition 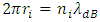 is not reasonable to automatically interlock with the velocity
is not reasonable to automatically interlock with the velocity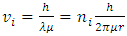 | (2.3) |
How can the electrons know well the exact distance to jump from the current energy level and de Broglie condition 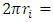
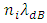 to the new energy level that is exactly fit the new de Broglie matter wave condition
to the new energy level that is exactly fit the new de Broglie matter wave condition 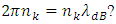 Another problem lies in that, is the de Broglie’s matter standing wave condition formed before the energy level transition or after the transition?
Another problem lies in that, is the de Broglie’s matter standing wave condition formed before the energy level transition or after the transition?
1.3. Only Continuous Cycles of Electron Energy Level Transitions can Produce Radiations with Same Frequency (That is Measureable)
One single unidirectional energy level transition produces only one single EM pulse of radiation that is immeasurable!Only continuous multiple back and forth energy level transitions can produce same frequency measurable cycles of radiations! As shown in Fig.3.1.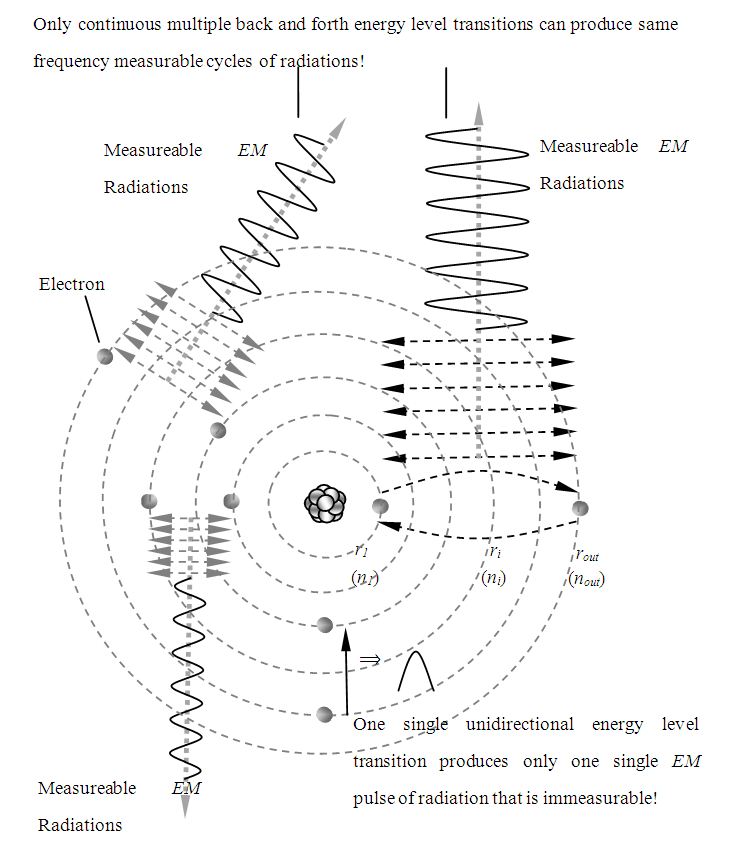 | Figure 3.1. Continuously moving electrons generate EM radiation |
And, when the electron transit from ith energy level to kth energy level most likely will emit k – i number cycles of radiation. In other words, when the electron transit from one level to next level, it will emit one cycle radiation, thus travelling k –I energy levels will emit k –i cycles of radiation. Prevailing explanation of energy level transition of electrons is superficial hypothesis.Do we have method or tools that can measure the frequency ν of one single energy level transition of one electron currently?In the measurement of quanta energy of the photoelectric effect, because we preset a monochromatic light beam before hand, thus we can measure continuous photoelectric current therefore to analyze to know the frequency and energy hf or h and f.Therefore, the explanation of one single energy transition generates a photon or generates a quanta energy hf is a nerd's imagination out of reality practices.The heat radiation and EM wave are continuous and we can measure only the electron continuously emitted EM radiations.If one single energy level transition of electron or other kind of single quanta energy of electron is measurable, then no need Heisenberg’s uncertainty rules and then quantum entanglement is mature technology already!
1.4. Derivation of a Most Possible Novel Atom Model Logically According to Actual Situation and Conditions of Electrons
1.4.1. Several Quantum Mechanics Related Key Points must be Clarified
1.4.1.1 There are existed several crucial problems in Bohr’s hydrogen atom model. One is the circular electron de Broglie’s wave being absent of restoring force to maintain the wave. The second problem is the circular electron de Broglie’s wave not having standing wave condition or not having boundaries of standing wave.The third problem is there being no mechanism of interconnection and coexistence between the electron de Broglie’s wave and energy level transition i.e.,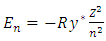 | (4.1) |
and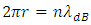 | (4.2) |
are not interlocked.In other words, how can an electron stably stay in an energy level, and instantaneously stably stay in next energy level after energy level transition. 1.4.1.2 The velocity of the electron during the energy level transition must be a sinusoidal function curve, not a square pulsation. Because any velocity of object with mass cannot reach zero instantaneously except it is inelastic collision onto a rest infinite mass.1.4.1.3 It is contradiction that frequency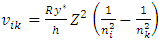 | (4.3) |
is higher, the electron instead of travelling shorter distance rather travelling longer distance to transit energy levels (ni-nk). Normally, travelling longer distance should spend longer time, and vice the versa.When temperature T is increased, kinetic energy is increased, velocity is increased, then hν is increased, there 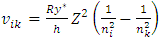 | (4.4) |
is increased, the distance between energy levels i.e., the distance between ni and nk is increased. How is the frequency higher while the distance of electrons spending longer time to travel longer distance?1.4.1.4 If the wave of radiation of electron is measurable, it must be continuous multiple cycles of same frequency ν.1.4.1.5 When charged particle is accelerating and decelerating repeatedly then can generate EM wave radiation. No acceleration-deceleration motion of charged particle (such as electrons) no EM radiation will be generated.If there is periodical acceleration-deceleration motions of charged particle, there must be two periodical forces pair in opposite directions (e.g., repulsive force and attractive force exerted on electrons) to act the charged particles accelerating and decelerating repeatedly.
1.4.2. Logically Deducing the Behaviors of Electrons According to Above Clarified Key Points
We logically derive several situation and behaviors of electron in an atom:1.4.2.1 An energy level that an electron can stably stay must be an equilibrium point of repulsive force and attractive force, and the transient velocity of electron energy level transition must be sinusoidal function not a square pulsation.1.4.2.2 One of the two periodical forces pair in opposite directions that exerted on electrons must be distributed in standing wave type in radial direction. Then the intersection spherical surface of the two opposite directional forces is the equilibrium position of the electrons and energy levels. 1.4.2.3 When temperature T is increased, kinetic energy is increased, hν is increased, then the velocity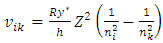 | (4.5) |
is increased higher, however, the distance between ni and nk is increased. However, since the time interval 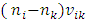 is proportional to the transition of longer distance, therefore the transient velocity must undergo ni - nk cycles of accelerate and decelerate changes thus to generate multiple cycles of EM radiation that can be measured. 1.4.2.4 The electron energy level transitions must be continuous back and forth multiple cycles thus generate same frequency ν continuously.1.4.2.5 The periodical acceleration-deceleration of electrons is due to two periodical forces pair in opposite directions exerted on electrons. They are thermal repulsive force [5] and Coulomb attractive force.
is proportional to the transition of longer distance, therefore the transient velocity must undergo ni - nk cycles of accelerate and decelerate changes thus to generate multiple cycles of EM radiation that can be measured. 1.4.2.4 The electron energy level transitions must be continuous back and forth multiple cycles thus generate same frequency ν continuously.1.4.2.5 The periodical acceleration-deceleration of electrons is due to two periodical forces pair in opposite directions exerted on electrons. They are thermal repulsive force [5] and Coulomb attractive force.
1.4.3. Derivation of a Most Possible Novel Atom Model Logically According to Above Clarified Key Points
We logically derive several situation and behaviors of electron in an atom:1.4.3.1 According to the previous discovery of universal thermal repulsive force [5], when the electron stably stays on an equilibrium point of an energy level, it is exerted by thermal repulsive force and Coulomb attractive force. The position of electron staying is one of equilibrium point (exactly say equilibrium spherical surface) of the repulsive force and attractive force.1.4.3.2 The electric field of the nucleus must be distributed in standing wave type in radial direction, as shown in Fig.4.1.(And the standing wave type distribution is both stationary in transversal direction and longitudinal direction)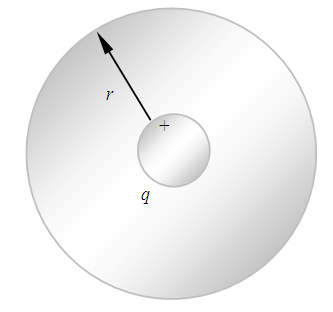 | Figure 4.1. Spherical Gaussian surface |
Gauss’s law describes, consider a positive point charge q located at the center of a sphere of radius r, from equation 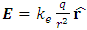 (where
(where 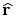 is a unit vector directed from the charge to the point in question), we know that the magnitude of the electric field everywhere on the surface of the sphere is
is a unit vector directed from the charge to the point in question), we know that the magnitude of the electric field everywhere on the surface of the sphere is 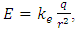 the field lines are directed radially outward and hence perpendicular to the surface at every point on the surface.A spherical Gaussian surface of radius r surrounding a point charge q, when the charge is at the center of the sphere, the electric field is everywhere normal to the surface and constant in magnitude. 1.4.3.3 The novel electric field of nucleus (or point charged particle) is very different from Gaussian’s model.Based on previously clarified key points, the electric field of the nucleus is different from Gaussian’s description: instead, the electric field of the nucleus must be distributed in standing wave type in radial direction, as shown in Fig.4.2.
the field lines are directed radially outward and hence perpendicular to the surface at every point on the surface.A spherical Gaussian surface of radius r surrounding a point charge q, when the charge is at the center of the sphere, the electric field is everywhere normal to the surface and constant in magnitude. 1.4.3.3 The novel electric field of nucleus (or point charged particle) is very different from Gaussian’s model.Based on previously clarified key points, the electric field of the nucleus is different from Gaussian’s description: instead, the electric field of the nucleus must be distributed in standing wave type in radial direction, as shown in Fig.4.2.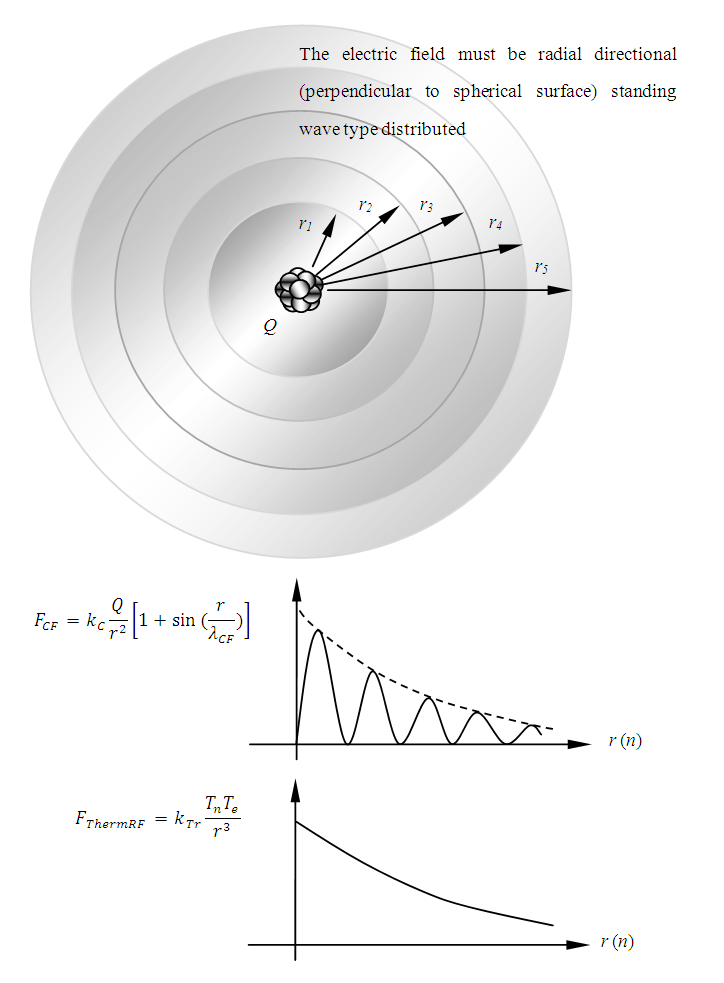 | Figure 4.2. Nucleus electric field being distributed in standing wave type in radial direction |
The thermal repulsive force is expressed as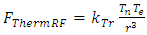 | (4.6) |
where r is radius, Tn is temperature of nucleus, Te is temperature of electron, 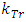 is coefficient.The Coulomb attractive force is expressed as
is coefficient.The Coulomb attractive force is expressed as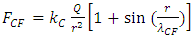 | (4.7) |
where r is radius, Q is quantity of electric charge of nucleus, 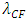 is wavelength of the electric field standing wave of the nucleus,
is wavelength of the electric field standing wave of the nucleus, 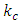 is coefficient.(The hypothesis or postulate of the standing wave type distributed electric field of the electric charge particle can adequately explain the wave behaviors of electron beam. The uniform electric field manifests no standing wave property, which is due to the homogenization of multiple fields of multiple charges).The wavelength
is coefficient.(The hypothesis or postulate of the standing wave type distributed electric field of the electric charge particle can adequately explain the wave behaviors of electron beam. The uniform electric field manifests no standing wave property, which is due to the homogenization of multiple fields of multiple charges).The wavelength 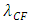 of the standing wave type distributed electric field of the electric charge of nucleus Q (or point electric charge particle) is inversely proportional to the electric charge magnitude or quantity of Q (that is why the equation of
of the standing wave type distributed electric field of the electric charge of nucleus Q (or point electric charge particle) is inversely proportional to the electric charge magnitude or quantity of Q (that is why the equation of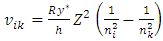 | (4.8) |
has a coefficient of charge number Z for larger mass nuclear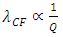 | (4.9) |
For a certain quantity of Q, the wavelength 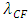 of the E field is a constant.Therefore, the electron travels from
of the E field is a constant.Therefore, the electron travels from 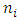 to
to 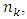 the energy level transitions start from
the energy level transitions start from 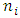 to
to 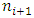 through
through 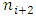 to
to 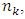 the electron experiences many energy levels transitions; every one of them generate a cycle of EM radiation of frequency
the electron experiences many energy levels transitions; every one of them generate a cycle of EM radiation of frequency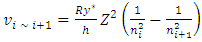 | (4.10) |
For hydrogen nuclear the wavelength 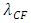 is the minim standing wave wavelength of charges
is the minim standing wave wavelength of charges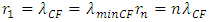 | (4.11) |
For larger mass nuclear with charge number Z, the standing wavelength is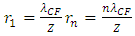 | (4.12) |
1.4.3.4 We can set the thermal repulsive force field as positive values, set the Coulomb attractive force field as negative values, then, draw both graphic curves in one diagram to illustrate the relationship graphically, as shown in Fig.4.3.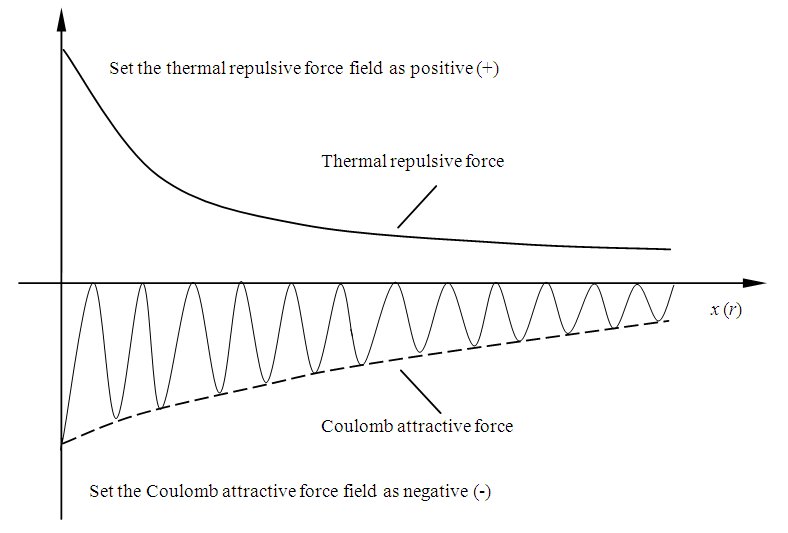 | Figure 4.3. Set repulsive force as positive and attractive force as negative |
Combine the two curves or find the sum of the thermal repulsive force and the Coulomb attractive force and draw the graphic curves in one diagram, we can see the total actual force function acted on electrons, as shown in Fig.4.4.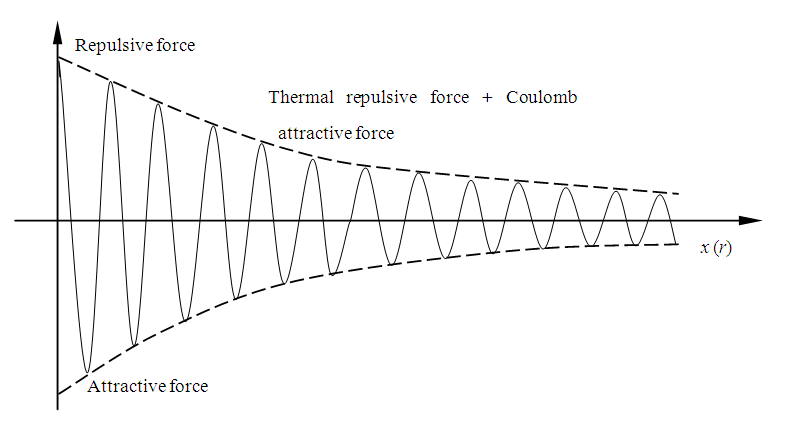 | Figure 4.4. Combine repulsive force and attractive force |
1.4.3.5 According to the Equation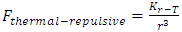 | (4.13) |
of discovered thermal repulsive force provided in Section 1 of this paper, we can calculate the energy change of electrons. Since 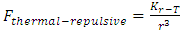 | (4.14) |
then the energy change of a hydrogen atom that goes from an initial state of quantum number ni to a final state of quantum number nk is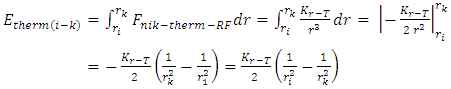 | (4.15) |
Substituting equation (4.11) 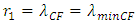 and
and 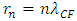 into (4.15), obtains
into (4.15), obtains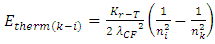 | (4.16) |
For larger mass nucleus with charge number Z, the standing wavelength is expressed as equation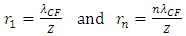 | (4.17) |
Substituting equation (4.17) into (4.16), obtains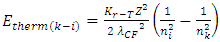 | (4.18) |
In microscopic field, the Coulomb force could be also inversely proportional to 3th powers of distance or radius r, thus the Coulomb force could be expressed as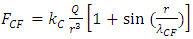 | (4.19) |
Thus the energy change being respected to electric field (or Coulomb force) could be also the similar format of thermal repulsive force field.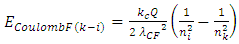 | (4.20) |
Therefore the total energy change should be the same format with the prevailing equation: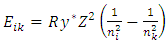 | (4.21) |
1.5. The Photoelectric Effect Actually is the Forward Energy Level Transitions of Electrons at Extreme Condition
The photoelectric effect actually is the continuous energy level transitions of electrons being re-excited by EM wave radiations. The energy of light wave absorbed by electron start to move forward to firstly cancel or overcome the so called work function Wa,.Therefore the prevailingly so called work function is actually the work done by electron absorbed EM wave energy to overcome the attractive force of the first half cycle of the fluctuate force fields, as shown in Fig.5.2.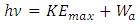 | (5.1) |
Then the electron is continuously transiting through the standing wave like distributed repulsive forces and attractive forces (i.e., the energy levels) to escape from the atom, as shown in Fig.5.1.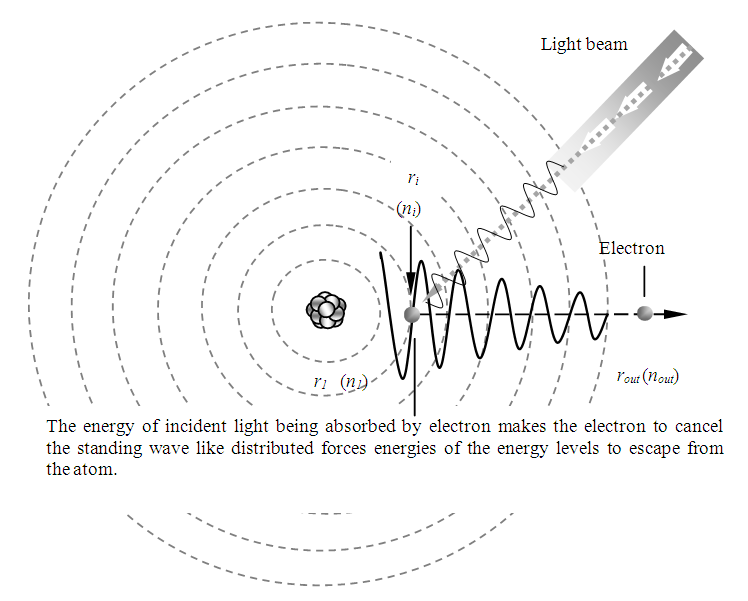 | Figure 5.1. Actual situation of photoelectric effect |
When the energy 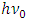 of the EM wave being absorbed by electron is larger than the work-function
of the EM wave being absorbed by electron is larger than the work-function 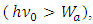 the electron will move forward and then firstly encounter and cancel the attractive force waveform zone that actually is equal to the work function Wa. That is why
the electron will move forward and then firstly encounter and cancel the attractive force waveform zone that actually is equal to the work function Wa. That is why 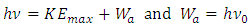 | (5.2) |
and in practices, whenever the critical frequency 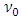 determined quanta energy being larger than hν, is absorbed by electron, then it is higher enough to firstly cancel the portion of potential energy of the attractive force and in turn continuously absorbs and cancels the following fluctuated attractive and repulsive forces energy thus to escape from the bind of the atom.Because when the electron passes through the following fluctuated plus-minus forces wave zone, although the electron is accelerated and decelerated periodically, it is almost not doing work and no work is done on it. As shown in Fig.5.2.
determined quanta energy being larger than hν, is absorbed by electron, then it is higher enough to firstly cancel the portion of potential energy of the attractive force and in turn continuously absorbs and cancels the following fluctuated attractive and repulsive forces energy thus to escape from the bind of the atom.Because when the electron passes through the following fluctuated plus-minus forces wave zone, although the electron is accelerated and decelerated periodically, it is almost not doing work and no work is done on it. As shown in Fig.5.2.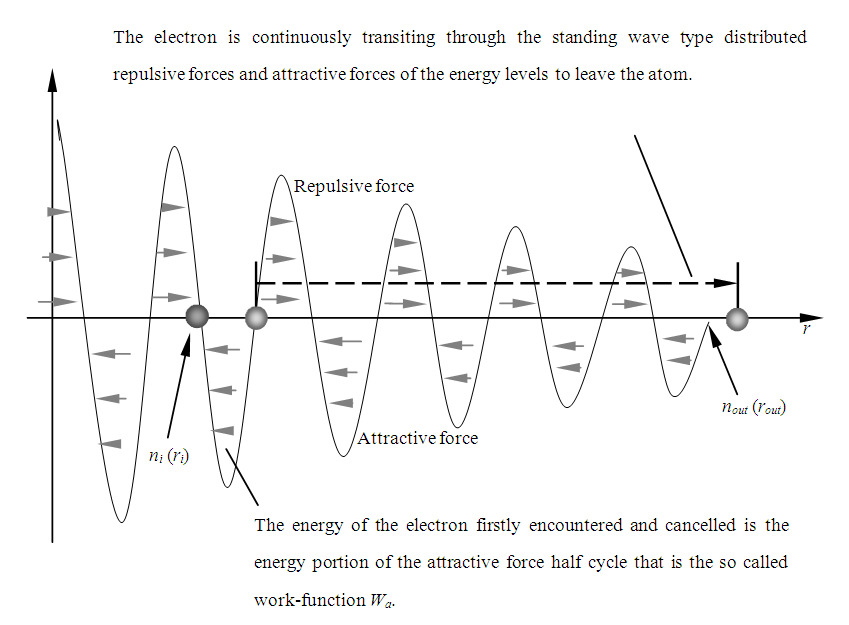 | Figure 5.2. Electron cancels the work-function Wa to escape the atom |
1.6. The (Man-Made) X-ray Actually is the Backward Energy Level Transitions of Electrons at Extreme Condition
The (man-made) X-ray emission process is actually a reversal process of photoelectric effect. It is the continuous backward energy level transitions of electrons due to electron absorbed kinetic energy. When the electron acquires a higher speed and moves toward the nucleus, it is continuously transiting through the standing wave like distributed repulsive forces and attractive forces (i.e., the energy levels) backwardly to enter into the atom, as shown in Fig.6.1.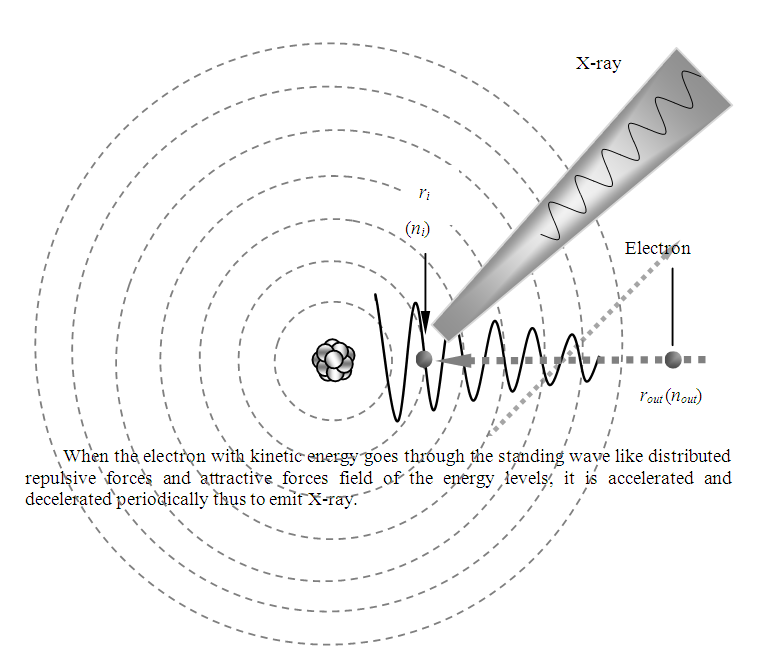 | Figure 6.1. Real situation of X-ray emission |
When the electron passes through the fluctuated standing wave type distributed plus-minus forces wave zone, the electron is accelerated and decelerated periodically by the repulsive and attractive forces, thus the electron generates the X-ray EM wave radiation, as shown in Fig.6.2.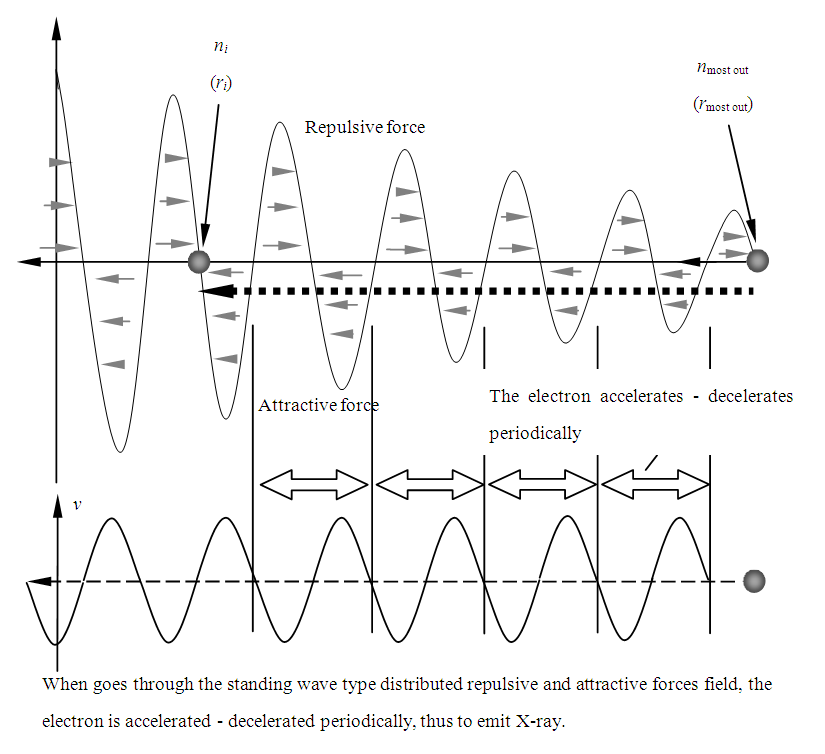 | Figure 6.2. Illustrate the generation of X-ray in more details |
1.7. The Actual Movement of Electrons Inside Atom to Generate EM Wave (Light Wave) Radiations
1.7.1 When electron is exerted external force or absorbs energy converted to kinetic energy, it will pass through the fluctuated standing wave type distributed repulsive force and attractive force fields of the nucleus, it will cause the oscillation of ether medium to produce EM wave.1.7.2 Before produce and emit EM wave radiation, the electrons are stayed in the repulsive force and attractive force equilibrium positions or surface, as shown in Fig.7.1.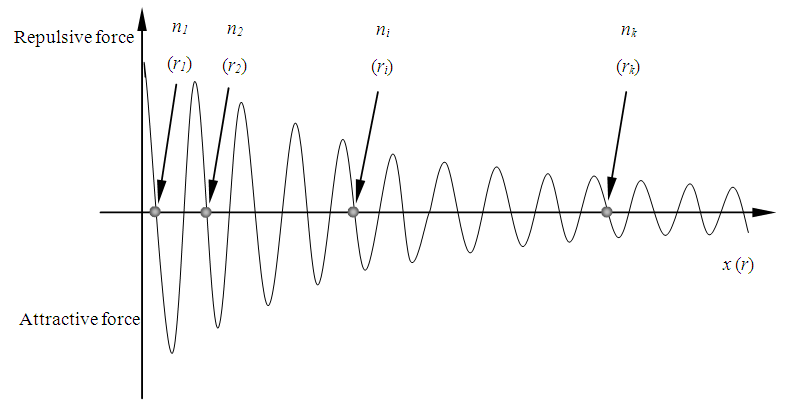 | Figure 7.1. The electron stays in equilibrium position of attractive force and repulsive force |
At the positions, the attractive force will exert on the electron to draw it move backward when it moves forward, and the repulsive force will exert on electron when it moves backward to repel it move forward, as shown in Fig.7.2. 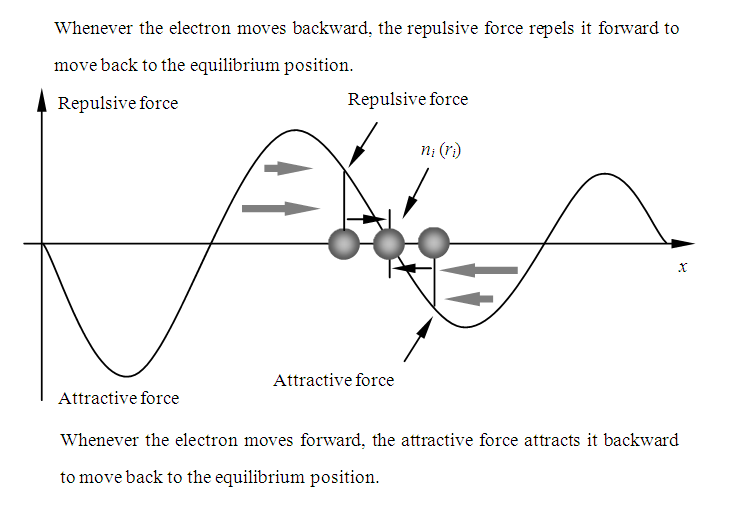 | Figure 7.2. Partial enlarged diagram to illustrate |
1.7.3 When temperature T is increased, thermal repulsive force is increased, the curve of the repulsive force will shift upper, then the repulsive force will exert on electron, the electron will move forward, if the force is higher enough, the electron will overcome the first attractive force half cycle, then travel from energy level ni to energy level nk During the transient, the velocity of electron accelerate and decelerate nk- ni times thus to generate and emit (nk - ni)cycles of EM wave radiations, as shown in Fig.7.3.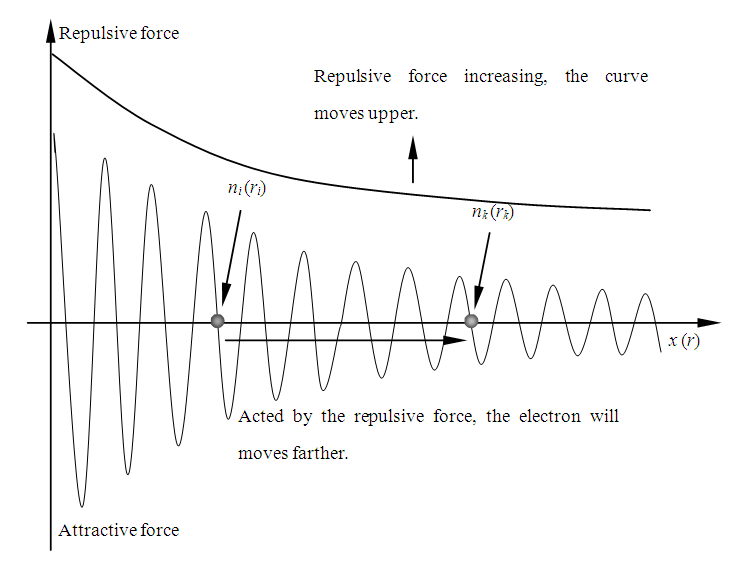 | Figure 7.3. Electron moves when temperature is increased |
7.4 When temperature T is decreased, thermal repulsive force is decreased, the curve of the repulsive force will shift downer, then the attractive force will exert on electron, the electron will move backward, if the force is higher enough, the electron will travel from energy level ni to energy level nk. During the transient, the velocity of electron accelerates and decelerates nk- ni times thus to generate and emit (nk - ni) cycles of EM wave radiations, as shown in Fig.7.4.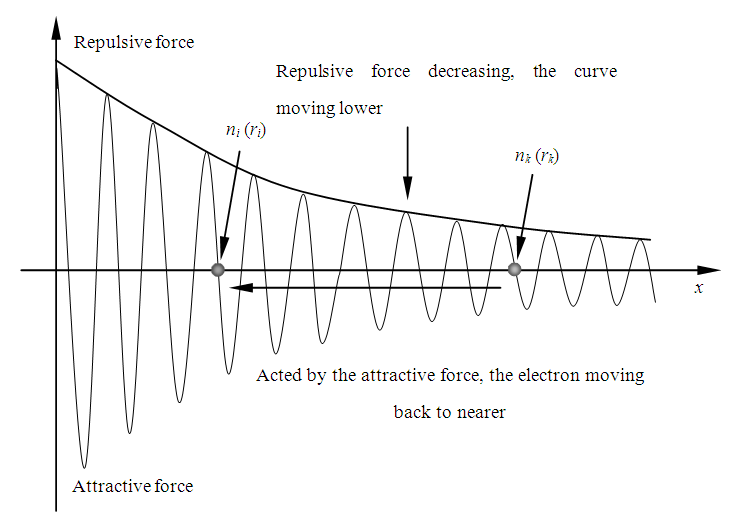 | Figure 7.4. Electron moves when temperature is decreased |
The electron energy levels transitions must continuously travel back and forth multiple cycles thus generate same frequency ν continuously then it is measurable.When the electron moves back and forth between nk to ni and ni to nk repeatedly, the wave radiation of frequency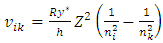 | (7.1) |
is measurable and be measured. As shown in Fig.7.5.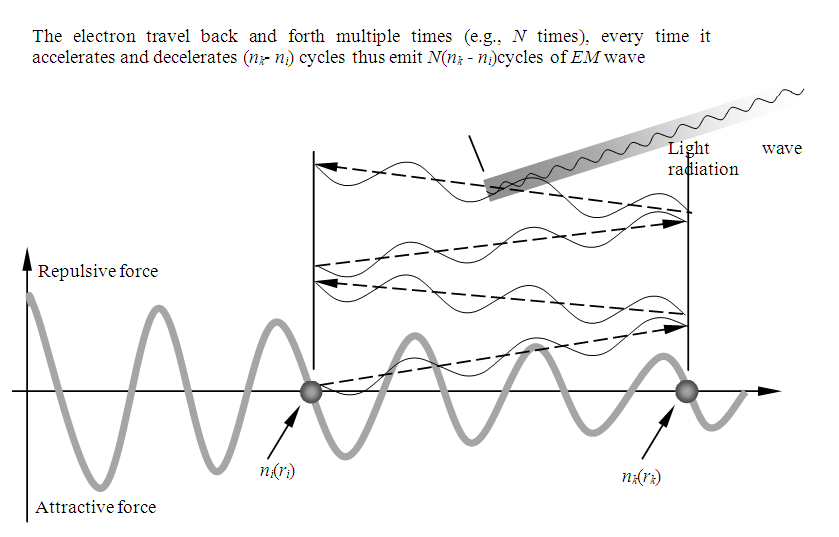 | Figure 7.5. Partial enlarged diagram to illustrate details |
E.g., for i = i to i = i+4, the velocity 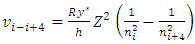 | (7.2) |
can be re-expressed as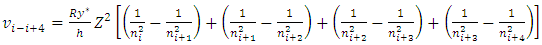 | (7.3) |
Simplified obtains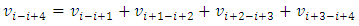 | (7.4) |
Every item is multiplied by Planck’s constant h obtains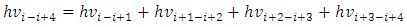 | (7.5) |
Therefore the energy change is 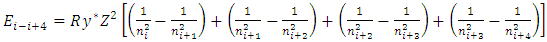 | (7.6) |
Simplified yields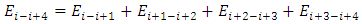 | (7.7) |
If 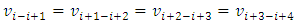 | (7.8) |
Or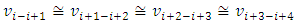 | (7.9) |
then 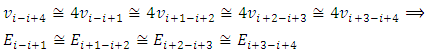 | (7.10) |
Therefore, the electron emitting cycles EM wave of same frequency 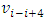 is possible.When the force enveloping curve is declining in radial direction
is possible.When the force enveloping curve is declining in radial direction 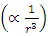 , the magnitude of the half cycle of positive repulsive force is larger than the magnitude of the half cycle of negative attractive force, the difference is almost exactly equal to the energy of one cycle of EM wave, then, the average velocity v of the electron in the 4 transitions of energy levels is almost same.Since frequency is
, the magnitude of the half cycle of positive repulsive force is larger than the magnitude of the half cycle of negative attractive force, the difference is almost exactly equal to the energy of one cycle of EM wave, then, the average velocity v of the electron in the 4 transitions of energy levels is almost same.Since frequency is | (7.11) |
The wavelength 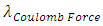 of Coulomb force field is constant, if the average velocity
of Coulomb force field is constant, if the average velocity 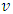 of electron is same, then the equations of the frequencies
of electron is same, then the equations of the frequencies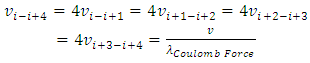 | (7.12) |
is tenable. The electron will emit (k - i) cycles of same frequency 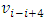 EM wave radiation during the transient of energy levels transition of level ni to level nk .Therefore, it is explainable why frequency is higher, the electron travel longer distance rather than shorter distance. Because, during the longer distance travelling, the electron accelerates-decelerates (nk ̶̶ ni) times, thus emits (nk ̶̶ ni) cycles of EM wave not only one transition emit only one cycle wave.
EM wave radiation during the transient of energy levels transition of level ni to level nk .Therefore, it is explainable why frequency is higher, the electron travel longer distance rather than shorter distance. Because, during the longer distance travelling, the electron accelerates-decelerates (nk ̶̶ ni) times, thus emits (nk ̶̶ ni) cycles of EM wave not only one transition emit only one cycle wave.
2. Conclusions
Based on above analysis, we can derive a novel atom model, in which the nucleus-electrons system is in static equilibrium states of universal static thermal repulsive conservative force and Coulomb force.The positions of energy levels of atom should be spatial spherical surfaces of equilibriums between thermal repulsive conservative forces and Coulomb forces. The electrons are laid statically or moving slowly in the equilibrium surface when no radiation energy is absorbed.When electron absorbs energy of external radiation, it will cancel the work-function Wa which is a half cycle portion of standing wave like distributed attractive-repulsive forces field and in turn moves in the forces field of atom to escape from the atom or generate radiation of different wavelength such as light waves.When electron is incident into atom with kinetic energy, it will be accelerated-decelerated periodically by the field of attractive force and repulsive force of atom to emit radiation (X-ray).
References
| [1] | David Halliday, Robert Resnick, Jearl Walker: Fundamental of Physics (8th edition). John Wiley & Sons, Inc 2008. |
| [2] | Wolfgang Demtröder: Atoms, Molecules and Photons. Springer 2010. |
| [3] | Beiser Arthur: Concepts of modern physics, 6th edition. McGraw-Hill Higher Education 2003. |
| [4] | Alan Jeffrey: Handbook of Mathematical formulas and integrals Second Edition. Academic Press 2000. |
| [5] | Liu Kedian: Discovery Of A Universal Static Thermal Repulsive Force. American Academic Press 2022. |


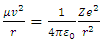
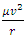 is only the tangential component (vx). The velocity vy of the vertical direction y is not taken into account. One single matter or electron has no conditions to form a standing wave or standing matter wave. There are no boundary conditions of standing wave for a single electron, let alone multiply electrons in same orbit. Therefore Bohr’s model is groundless.The electrons and the nucleus must be in relative static stable equilibrium state at different (potential) energy levels, rather than in kinetic energy levels.
is only the tangential component (vx). The velocity vy of the vertical direction y is not taken into account. One single matter or electron has no conditions to form a standing wave or standing matter wave. There are no boundary conditions of standing wave for a single electron, let alone multiply electrons in same orbit. Therefore Bohr’s model is groundless.The electrons and the nucleus must be in relative static stable equilibrium state at different (potential) energy levels, rather than in kinetic energy levels.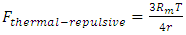
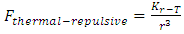
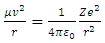
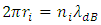
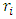 is radius of energy level i,
is radius of energy level i, 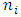 is quantum number of energy level i,
is quantum number of energy level i, 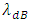 is de Broglie’s wavelength) is purely an imagination. It does not matter to temporarily believe it being true; however, the condition
is de Broglie’s wavelength) is purely an imagination. It does not matter to temporarily believe it being true; however, the condition 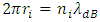 is not reasonable to automatically interlock with the velocity
is not reasonable to automatically interlock with the velocity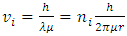
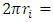
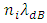 to the new energy level that is exactly fit the new de Broglie matter wave condition
to the new energy level that is exactly fit the new de Broglie matter wave condition 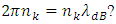 Another problem lies in that, is the de Broglie’s matter standing wave condition formed before the energy level transition or after the transition?
Another problem lies in that, is the de Broglie’s matter standing wave condition formed before the energy level transition or after the transition?
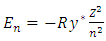
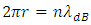
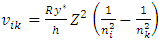
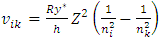
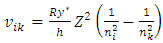
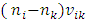 is proportional to the transition of longer distance, therefore the transient velocity must undergo ni - nk cycles of accelerate and decelerate changes thus to generate multiple cycles of EM radiation that can be measured. 1.4.2.4 The electron energy level transitions must be continuous back and forth multiple cycles thus generate same frequency ν continuously.1.4.2.5 The periodical acceleration-deceleration of electrons is due to two periodical forces pair in opposite directions exerted on electrons. They are thermal repulsive force [5] and Coulomb attractive force.
is proportional to the transition of longer distance, therefore the transient velocity must undergo ni - nk cycles of accelerate and decelerate changes thus to generate multiple cycles of EM radiation that can be measured. 1.4.2.4 The electron energy level transitions must be continuous back and forth multiple cycles thus generate same frequency ν continuously.1.4.2.5 The periodical acceleration-deceleration of electrons is due to two periodical forces pair in opposite directions exerted on electrons. They are thermal repulsive force [5] and Coulomb attractive force.
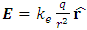 (where
(where 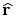 is a unit vector directed from the charge to the point in question), we know that the magnitude of the electric field everywhere on the surface of the sphere is
is a unit vector directed from the charge to the point in question), we know that the magnitude of the electric field everywhere on the surface of the sphere is 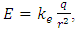 the field lines are directed radially outward and hence perpendicular to the surface at every point on the surface.A spherical Gaussian surface of radius r surrounding a point charge q, when the charge is at the center of the sphere, the electric field is everywhere normal to the surface and constant in magnitude. 1.4.3.3 The novel electric field of nucleus (or point charged particle) is very different from Gaussian’s model.Based on previously clarified key points, the electric field of the nucleus is different from Gaussian’s description: instead, the electric field of the nucleus must be distributed in standing wave type in radial direction, as shown in Fig.4.2.
the field lines are directed radially outward and hence perpendicular to the surface at every point on the surface.A spherical Gaussian surface of radius r surrounding a point charge q, when the charge is at the center of the sphere, the electric field is everywhere normal to the surface and constant in magnitude. 1.4.3.3 The novel electric field of nucleus (or point charged particle) is very different from Gaussian’s model.Based on previously clarified key points, the electric field of the nucleus is different from Gaussian’s description: instead, the electric field of the nucleus must be distributed in standing wave type in radial direction, as shown in Fig.4.2.
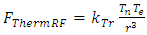
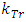 is coefficient.The Coulomb attractive force is expressed as
is coefficient.The Coulomb attractive force is expressed as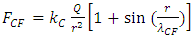
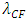 is wavelength of the electric field standing wave of the nucleus,
is wavelength of the electric field standing wave of the nucleus, 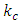 is coefficient.(The hypothesis or postulate of the standing wave type distributed electric field of the electric charge particle can adequately explain the wave behaviors of electron beam. The uniform electric field manifests no standing wave property, which is due to the homogenization of multiple fields of multiple charges).The wavelength
is coefficient.(The hypothesis or postulate of the standing wave type distributed electric field of the electric charge particle can adequately explain the wave behaviors of electron beam. The uniform electric field manifests no standing wave property, which is due to the homogenization of multiple fields of multiple charges).The wavelength 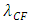 of the standing wave type distributed electric field of the electric charge of nucleus Q (or point electric charge particle) is inversely proportional to the electric charge magnitude or quantity of Q (that is why the equation of
of the standing wave type distributed electric field of the electric charge of nucleus Q (or point electric charge particle) is inversely proportional to the electric charge magnitude or quantity of Q (that is why the equation of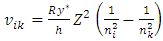
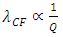
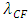 of the E field is a constant.Therefore, the electron travels from
of the E field is a constant.Therefore, the electron travels from 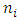 to
to 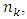 the energy level transitions start from
the energy level transitions start from 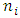 to
to 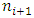 through
through 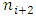 to
to 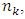 the electron experiences many energy levels transitions; every one of them generate a cycle of EM radiation of frequency
the electron experiences many energy levels transitions; every one of them generate a cycle of EM radiation of frequency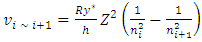
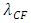 is the minim standing wave wavelength of charges
is the minim standing wave wavelength of charges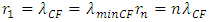
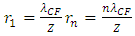


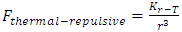
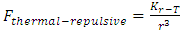
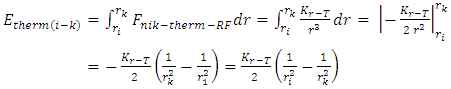
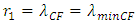 and
and 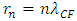 into (4.15), obtains
into (4.15), obtains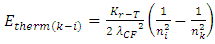
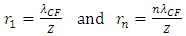
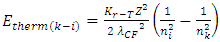
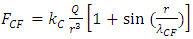
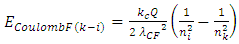
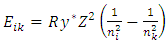
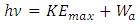

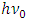 of the EM wave being absorbed by electron is larger than the work-function
of the EM wave being absorbed by electron is larger than the work-function 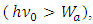 the electron will move forward and then firstly encounter and cancel the attractive force waveform zone that actually is equal to the work function Wa. That is why
the electron will move forward and then firstly encounter and cancel the attractive force waveform zone that actually is equal to the work function Wa. That is why 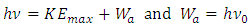
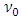 determined quanta energy being larger than hν, is absorbed by electron, then it is higher enough to firstly cancel the portion of potential energy of the attractive force and in turn continuously absorbs and cancels the following fluctuated attractive and repulsive forces energy thus to escape from the bind of the atom.Because when the electron passes through the following fluctuated plus-minus forces wave zone, although the electron is accelerated and decelerated periodically, it is almost not doing work and no work is done on it. As shown in Fig.5.2.
determined quanta energy being larger than hν, is absorbed by electron, then it is higher enough to firstly cancel the portion of potential energy of the attractive force and in turn continuously absorbs and cancels the following fluctuated attractive and repulsive forces energy thus to escape from the bind of the atom.Because when the electron passes through the following fluctuated plus-minus forces wave zone, although the electron is accelerated and decelerated periodically, it is almost not doing work and no work is done on it. As shown in Fig.5.2.






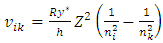

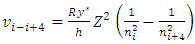
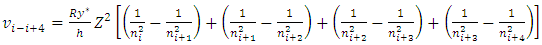
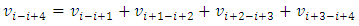
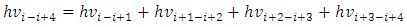
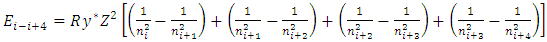
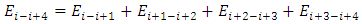
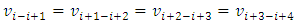
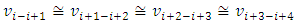
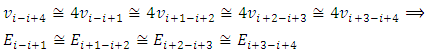
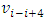 is possible.When the force enveloping curve is declining in radial direction
is possible.When the force enveloping curve is declining in radial direction 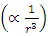 , the magnitude of the half cycle of positive repulsive force is larger than the magnitude of the half cycle of negative attractive force, the difference is almost exactly equal to the energy of one cycle of EM wave, then, the average velocity v of the electron in the 4 transitions of energy levels is almost same.Since frequency is
, the magnitude of the half cycle of positive repulsive force is larger than the magnitude of the half cycle of negative attractive force, the difference is almost exactly equal to the energy of one cycle of EM wave, then, the average velocity v of the electron in the 4 transitions of energy levels is almost same.Since frequency is
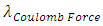 of Coulomb force field is constant, if the average velocity
of Coulomb force field is constant, if the average velocity 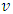 of electron is same, then the equations of the frequencies
of electron is same, then the equations of the frequencies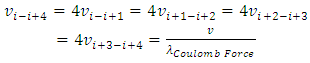
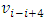 EM wave radiation during the transient of energy levels transition of level ni to level nk .Therefore, it is explainable why frequency is higher, the electron travel longer distance rather than shorter distance. Because, during the longer distance travelling, the electron accelerates-decelerates (nk ̶̶ ni) times, thus emits (nk ̶̶ ni) cycles of EM wave not only one transition emit only one cycle wave.
EM wave radiation during the transient of energy levels transition of level ni to level nk .Therefore, it is explainable why frequency is higher, the electron travel longer distance rather than shorter distance. Because, during the longer distance travelling, the electron accelerates-decelerates (nk ̶̶ ni) times, thus emits (nk ̶̶ ni) cycles of EM wave not only one transition emit only one cycle wave. Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML