-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Physical Chemistry
p-ISSN: 2167-7042 e-ISSN: 2167-7069
2019; 9(1): 1-7
doi:10.5923/j.pc.20190901.01

Thermal Study of Aluminum Trifluoromethyl Sulfonate as Effective Catalyst for the Polymerization of Epoxidized Linseed Oil
Ethnice Dehonor Márquez1, 2, Enrique Vigueras Santiago1, Susana Hernández López2
1Laboratorio de Desarrollo y Caracterización de Materiales Avanzados (LIDMA), Facultad de Química de Química de la Universidad Autónoma del Estado de México (UAEM), Paseo Tollocan Esquina con Paseo Colón, s/n. Moderna de la Cruz, Toluca, México
2Student in the Materials Science Program, UAEM
Correspondence to: Susana Hernández López, Student in the Materials Science Program, UAEM.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

It was studied the Aluminum Trifluoromethyl Sulfonate ((Al(OTf)3) as a very effective catalyst to polymerize Epoxidized Linseed Oil (ELO) in bulk, in a very wide range of temperatures, from room temperature (25°C) to the peak temperature of the exothermal curve of polymerization. Those temperatures and times of polymerization were depending on the amount of catalyst, which was evaluated in a very wide range of loads, from 0.5 x10-3 to 70 x10-3 molar percent. As an instance, a 55 x10-3 molar percent of catalyst showed at exothermic curve from 82 to 187°C with the peak temperature at 120.3°C. This preparation allows to ELO polymerize at 25°C in 24 hrs, at 60°C in 1hr and at 80°C in 20 min and only takes 7 min to polymerize at peak temperature. The study of the opening of the epoxy ring and polymerization of ELO was made by DSC and FTIR-HART analysis. Also was evidenced by 1H-NMR that no side reactions are promoted by this catalyst at least in the first minutes of reaction.
Keywords: Aluminum triflate, Oxirane aperture, Epoxidized linseed oil
Cite this paper: Ethnice Dehonor Márquez, Enrique Vigueras Santiago, Susana Hernández López, Thermal Study of Aluminum Trifluoromethyl Sulfonate as Effective Catalyst for the Polymerization of Epoxidized Linseed Oil, Physical Chemistry, Vol. 9 No. 1, 2019, pp. 1-7. doi: 10.5923/j.pc.20190901.01.
Article Outline
1. Introduction
- Epoxides (oxiranes) are functional groups reactive to a wide range of nucleophiles due to their angular tension and their C-O polar bond and are often used as starting materials and intermediates. Extrapolating to the epoxy resins, they can homopolymerize under acidic or basic conditions and/or with a variety of curing agents called curing agents or crosslinkers. Cured epoxy resins typically exhibit excellent thermal, mechanical, and thermal properties with low shrinkage properties so they are widely used as protectors, adhesives, composites, among others. On the other hand, with the demand for biopolymers and resins with practical applications, vegetable oils have been considered within renewable natural sources with high availability and low cost, as an excellent alternative of high synthetic potential to produce biodegradable polymers. [1,2]. Epoxidized vegetable oils (EVO) have been prepared by the epoxidation of C=C double bonds of fatty acids using peracids. There is a lot of literature about this reaction [3-8] due the EVOs [9] have been considered as intermediate reagents to generate other functional groups suitable [10-12] for the synthesis of polymers and they are industrially applied in the PVC industry as plasticizers. Among the most studied EVOs are epoxidized soybean oils (SBO) and epoxidized linseed oil (ELO) and are to date the only ones produced on an industrial scale. The content of oxirane groups depends on the type of vegetable oil (number of double bonds) [1] and the epoxidation method. EVOs can be polymerized by a cationic mechanism or can be used in UV curable systems efficiently at room temperature. The cationic polymerization performed at around 100°C may generate some lateral reactions [13,14]. While the Lewis acid catalyzed ring opening photo-polymerization is performed at room temperature using boron trihalides [15], superacids as fluorosulfonic acid [16,17] and strong acids [18], and salts of diaryliodonium [19]. However acids super acids and strong acids are toxics and/or corrosives and generates many side reactions as described in [16,17]. The aluminum trifluoromethanesulfonate or aluminum triflate, Al(OTf)3, a Lewis acid type catalyst that has been little studied for the opening of epoxy rings by amines [20] and by alcohols [21] in simple terminal epoxides (styrene oxide), finding that nucleophilic alcohol attacked the most hindered carbon atom of the epoxy ring (regioselective reaction) at temperatures of 0°C and in amounts on the order of 0.0005% by weight of the catalyst and achieved reaction yields of up to 97% with formation of oligomers quantity that was managed to minimize to traces increasing the amount of alcohol. When they performed the reaction with butylene oxide, they obtained an isomer ratio of approximately 50:50. When they performed the reaction on non-terminal epoxides but on small molecules such as cyclohexene oxide, 0.001% catalyst was required to generate the opening product in 85% yield. However, in performing the reaction on the cyclododecane oxide, they had to increase the amount of catalyst (0.04%) by 14 times and the yield was decreased to 49% yield. They attribute it to the effects of polarity difference between cyclododecane and alcohol (methanol) the difficulty of attack. Another study [15] made on different metal (Sc, Bi, La, Cu, Al, and Zn) triflate catalysts was on the transesterification reaction of Jatropha curcas L. oil with many alcohols. Aluminum trimethanesulfonate was the most effective catalysts rendering conversions of 99% followed by bismuth triflate. They also observed some methoxylation depending on the methanol excess and using Aluminum trimethanesulfonate as catalyst. In a previous work [22] that catalyst was tried to use in the functionalization of ELO with propargyl alcohol, obtaining in most cases the polymerization of ELO rather than functionalization. Even though EVOs are generally polymerized in formulations with other comonomers, hardeners and / or reinforcing agents [Lozada, 2009], one characteristic of thermal homopolymerization without catalysts is that it is carried out at temperatures around 200°C because of problems inherent in its structure as its flexibility and limited reactivity due to the high steric hindrance (internal oxiranes) in relation to the low amount of epoxy groups present (for ELO is around 6). This is why in this work it was determined to use Al(OTf)3 to study its effectiveness in the opening of 1,2-epoxy rings and polymerization of ELO in the absence of any external nucleophiles (amines or alcohols) in a range of amount of catalyst and temperatures below their exotherm curve. It was so effective that it becomes important to know the limit of temperature-time that we could keep the mixture once prepared in order to avoid a spontaneous polymerization below the range of temperatures observed under the exotherm curve. DSC and FTIR-HART spectroscopies allow to follow the degree of cure of reactive mixtures of ELO containing different amount of catalyst, Al(OTf)3.
2. Experimental
2.1. Materials
- Epoxidized linseed oil was previously prepared following the procedure described in [23]. It was characterized obtaining a 63% of epoxidation (4.0 of oxirane rings), and with a molecular weight of 873 g/mol (calculated by H1 NMR, 23). Al(OTf)3 99.9% trace metal bases, 474.19 g/mol molecular weight was purchased from Sigma-Aldrich, Co., all analytical grade solvents (THF, Acetone, CDCl3) were also purchased from Sigma-Aldrich, Co. and used as received. A stock solution of the catalyst in acetone was prepared (0.021 M) in order to take the necessary volumes to reach the molar percentage required: 0.5, 2.5, 5.0, 1.0, 15, 25, 35, 55 and 70 x10-3 mol% to study the polymerization reaction of ELO. Each mixture of ELO + Al(OTf)3 was prepared using 1g of ELO to which was added the required volume of the catalyst stock solution. Then it was stirred in an ultrasonic bath for one minute at 10°C. The acetone was evaporated under reduced pressure at room temperature for 2 h. Samples were characterized by FTIR spectroscopy and DSC/TGA analysis immediately after being prepared, and then kept under refrigeration at -20°C.
2.2. Methods
- Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis were performed on an SDT Q600 modulus from TA Instruments. Three type of analysis were made: (i) Both DSC and TGA were recorded to evaluate the thermal properties of pure ELO, Al(OTf)3 (named references) and the final polymers over the range of temperature from 25 to 570°C at 20°C/min under Nitrogen atmosphere (100 mL/min). It was used a 90L alumina DSC pan with alumina topper, an empty ceramic pan was used as reference (ii) For Isothermal experiments at temperatures lower than the respective cure curve (40 and 50°C, 60 80°C, 100 and 120°C). Experiments were performed using 5-7 mg of the reactive mixture (ELO+ Al(OTf)3) for detecting the exothermic crosslinking curve and the range of temperature associated to this polymerization process. iii) For characterizing the decomposition temperature (T10) of the products totally polymerized.FT-IR spectroscopic measurements were performed on a FTIR Prestige 21, Shimadzu spectrometer equipped with a horizontal attenuated total reflectance (HART) crystal made of diamond. FTIR spectroscopy was useful for (i) characterizing the references ELO and Al(OTf)3, and for characterizing the initial mixtures of ELO + Al(OTf)3 over the range of 4000 to 560cm-1. (ii) For monitoring the crosslinking reactions at temperatures at 25, 40, 50, 60, 80, 100 and 120°C from isothermal running on DSC. With this spectra was possible to quantify the degree of cure (equation 1) by normalize the spectrum to the carbonyl band (reference band) at 1740 cm-1 and by measure the area under the curve that form the pair of peaks centered at 821 and 798 cm-1 corresponding to the epoxy ring [24,25].
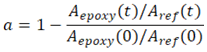 | (1) |
3. Results and Discussion
3.1. Characterization of References by DSC/TGA Analysis
- The characterization of both references: ELO and the catalyst were performed by DSC/ TGA. Figures 1 and 2 show the respective analysis. As it could be appreciate, DSC thermogram for pure ELO only shows the exothermic peak centered at 200°C and a highest exothermic peak corresponding at the decomposition process in the range of 350-425°C. Also ELO has a decomposition temperature, T10 at 389.6°C.
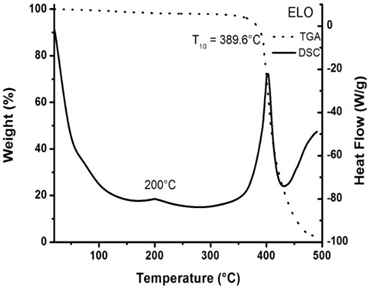 | Figure 1. Thermograms DSC and TGA for Epoxidized Linseed Oil (ELO) |
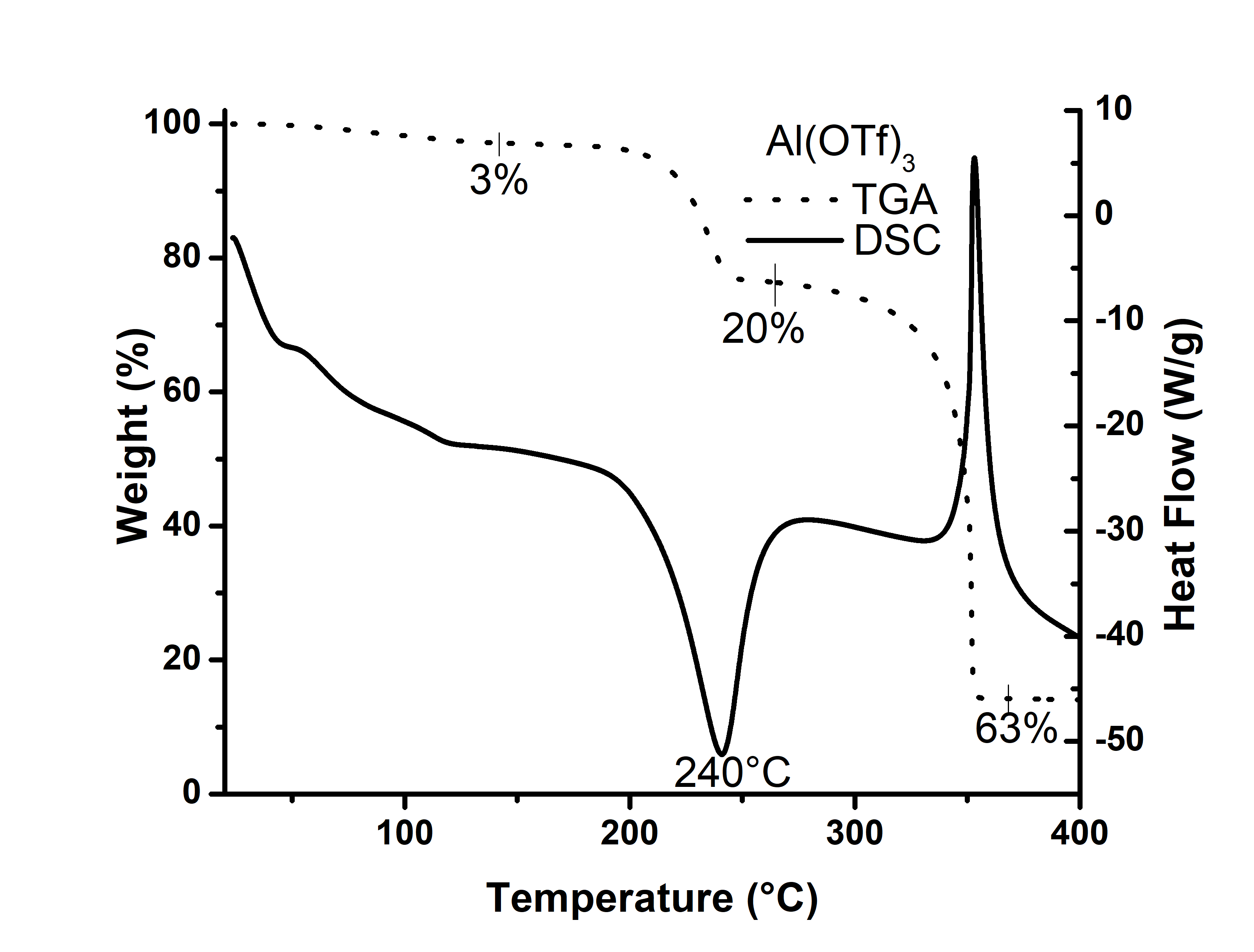 | Figure 2. Thermograms DSC and TGA for Aluminium Triflate |
3.2. Characterization by DSC and FTIR-HART Techniques of the Reactive Mixtures: ELO + Al(OTf)3
- DSC characterization of the initial mixtures and pure ELO is shown in Figure 3. Those thermograms were run at a heat rate of 20°/min. As could be seen, pure ELO (0% of catalyst) shows a lightly exotherm centered in 200°C (curve in green). As load of catalyst increases, that exotherm is shifted at lower temperatures. It is possible to appreciate three regions, the first defined by the peak temperature around 165°C (curves in blue) which corresponds to the lowest amount of catalyst (0.5 and 1 x 10-3 % mol). For the second region (curves in red) the peak temperatures are around the 120°C. These temperatures are for the mixtures with 2.5, 5, 11 and 16 x 10-3 % mol of catalyst. And finally the region with the lowest peak temperatures (in grey color) around 77°C for the highest amount of catalyst, 27, 35, 55 and 70 x 10-3 % mol. It is evident that cure temperature is diminished as the amount of catalyst is increased. From 200°C that requires the ELO without catalyst to approximately 77°C there is 100°C of difference. However, the curves for the third region are very small and for some compositions the exotherm is not longer visible. This fact is attributed to some oxirane aperture during the preparation, and even under refrigeration at -20°C. For mixture with 70 x10-3 % mol of catalyst there is no exotherm. These evidences were supported by FTIR, in Figure 4. Something that is sure is that at room temperature (23-25°C) they polymerize in around 30min. In fact for the mixture with 16 x 10-3 % mol resulted difficult to made a following of the cure process due to even in refrigeration each 12 h there was some degree of reaction as evidenced by FTIR and DSC. Due to the difficult to work with the compositions of the third region and with 16 x 10-3 % mol, we decided not to study them. All the other compositions were stables under refrigeration for long periods of time (weeks).
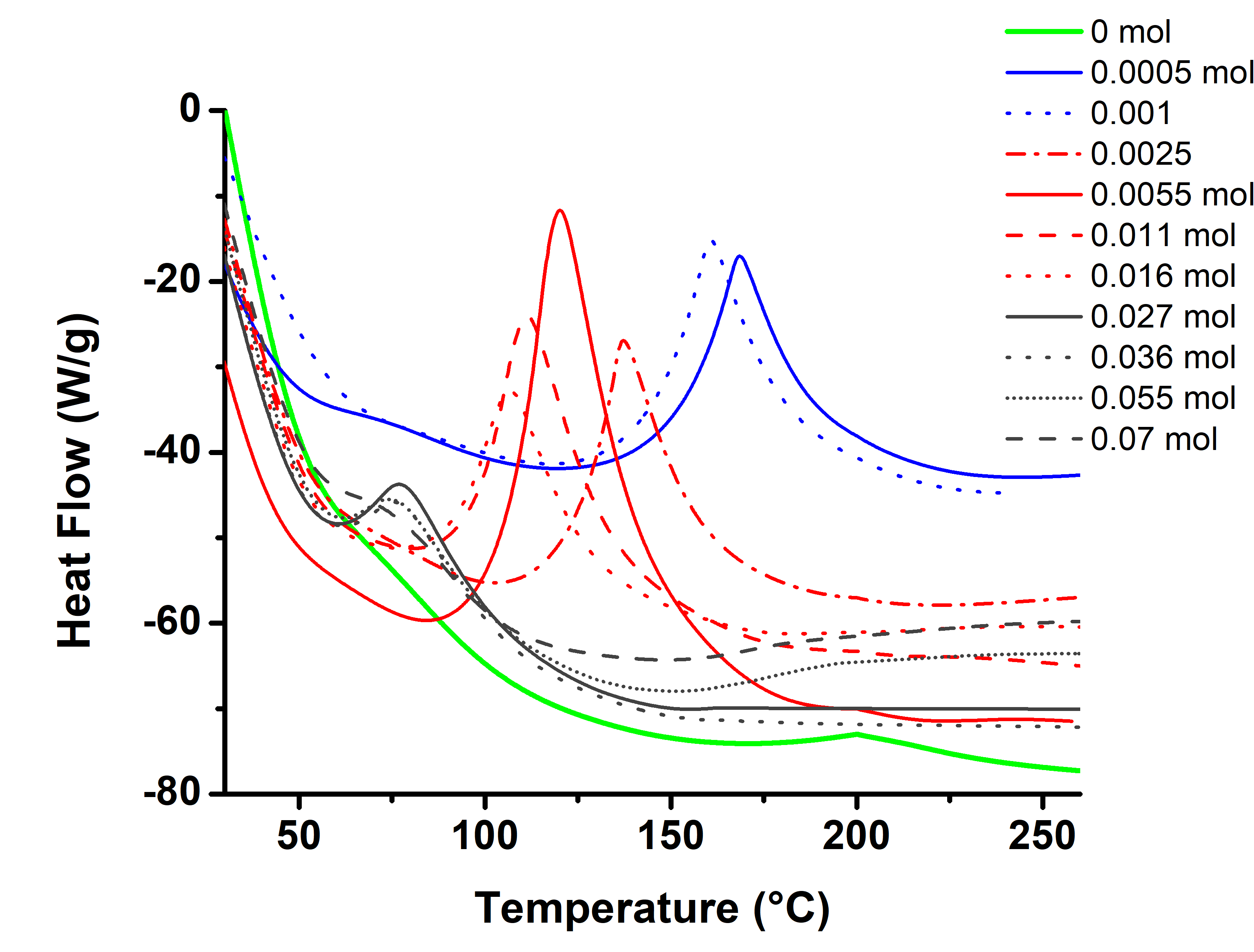 | Figure 3. Exhotherms from DCS curves of pure ELO and the mixtures respect to the mol percentage of catalyst |
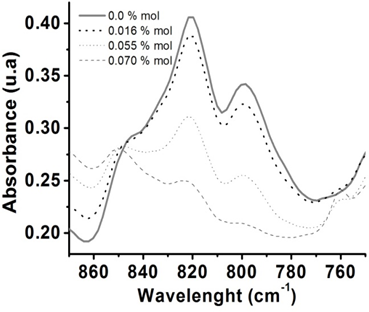 | Figure 4. Normalized FTIR-HART spectra in the range of the corresponding epoxy signal of ELO and the mixtures containing 16, 55 and 70 x 10-3 mol% of catalyst |
3.3. Cure under Isothermal Experiments by DSC
- The propose of this type of analysis was to detect a low temperature below the exothermic peak (Figure 1), at which is possible to cure and monitoring the reactive mixtures with different amount of catalyst. For isothermal analysis made using the DSC and temperatures of 40, 50, 60 and 80°C and in some mixtures 100 and 120°C, the range of programed time was from 5 min to several days. After cooling at 25°C, the same sample was reheated from 25 to 270°C in order to detect some residual curing. If would be one, a new sample of the same mixture was submitted at the same conditions but at longer times until confirm the nonexistence of residual curing. The same procedure was made for the other temperatures and for all the mixtures. The respective kinetics is showed in Figure 5.
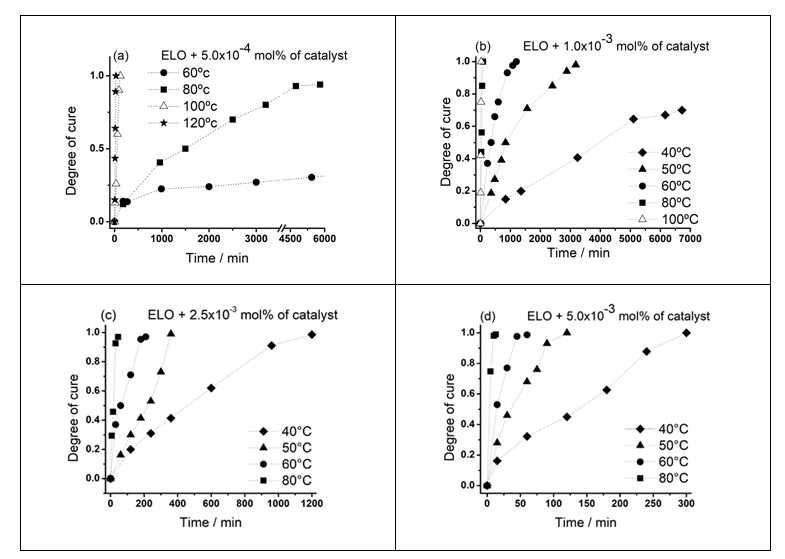 | Figure 5. Following of the Degree of cure from isothermal experiments for (a) 5.0 x 10-4, (b) 1.0 x 10-3, (c) 2.5 x 10-3, and 5.0 x 10-3 mol% of Al(OTf)3 |
3.4. Cure at 25°C Monitoring in situ by FTIR-HART Spectroscopy
- The analysis was performed directly on the liquid sample holder of the HART device in which 0.2 mg of sample was deposited. The spectrometer was programmed to generate a spectrum every 200 sweeps, at 4 cm-1 resolution in absorbance mode. Each sweep takes 4 min and all the time the sample is being irradiated by IR radiation during the experiment, keeping constant the temperature of the sample at 25°C. The experiment was stopped when the signal corresponding to the vibration of the epoxy ring vanished. Only the complete cured samples (confirmed first by FT-IR) were analyzed by DSC. An illustration of how this analysis was performed is exemplified in Figure 6 for the mixture ELO + 5.0x10-3 mol% of catalyst. It is possible to appreciate the degree of cure with the time.
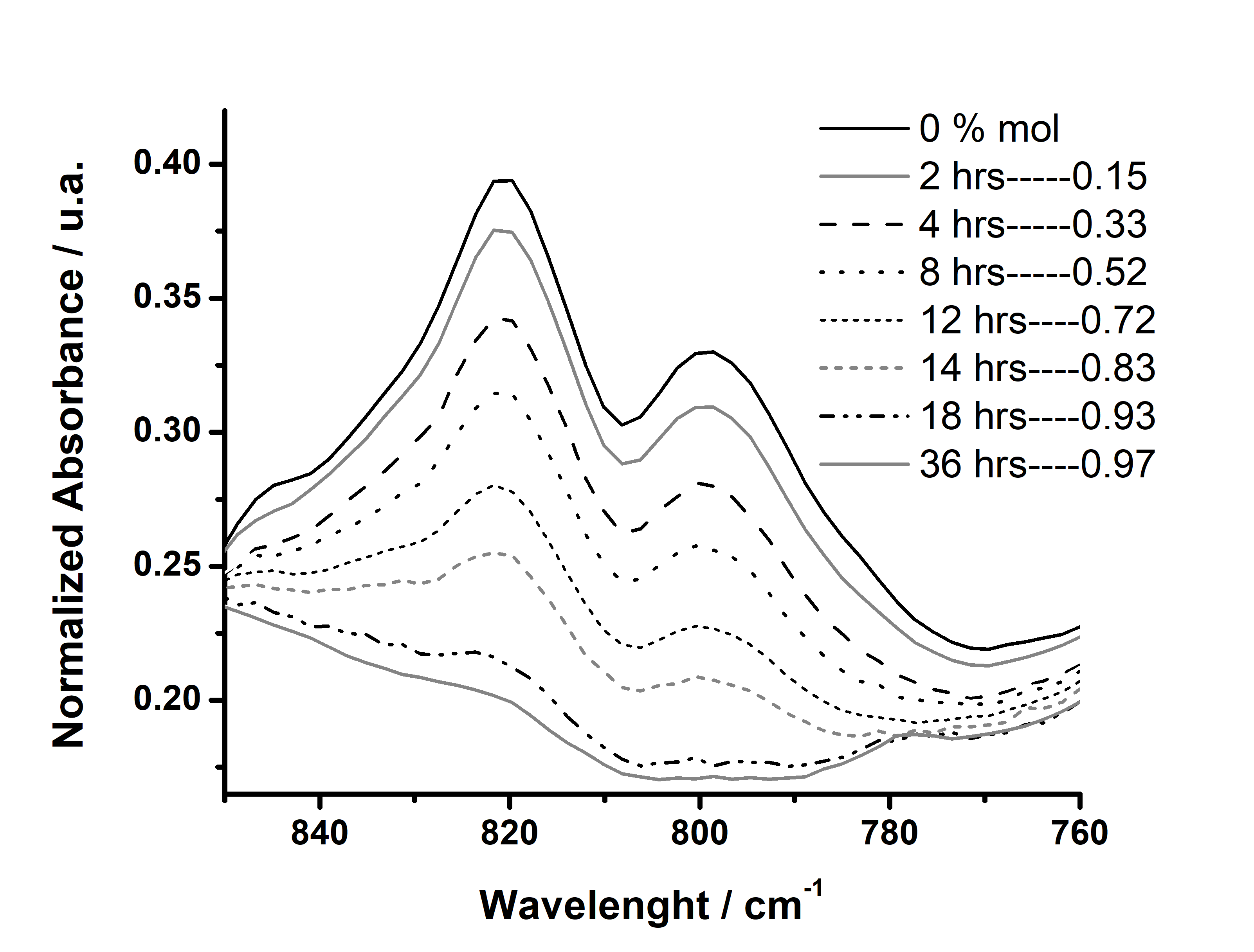 | Figure 6. Cure monitoring by FT-IR in situ at 25°C for ELO + 5.0 x 10-3 % mol of Al(OTf)3 |
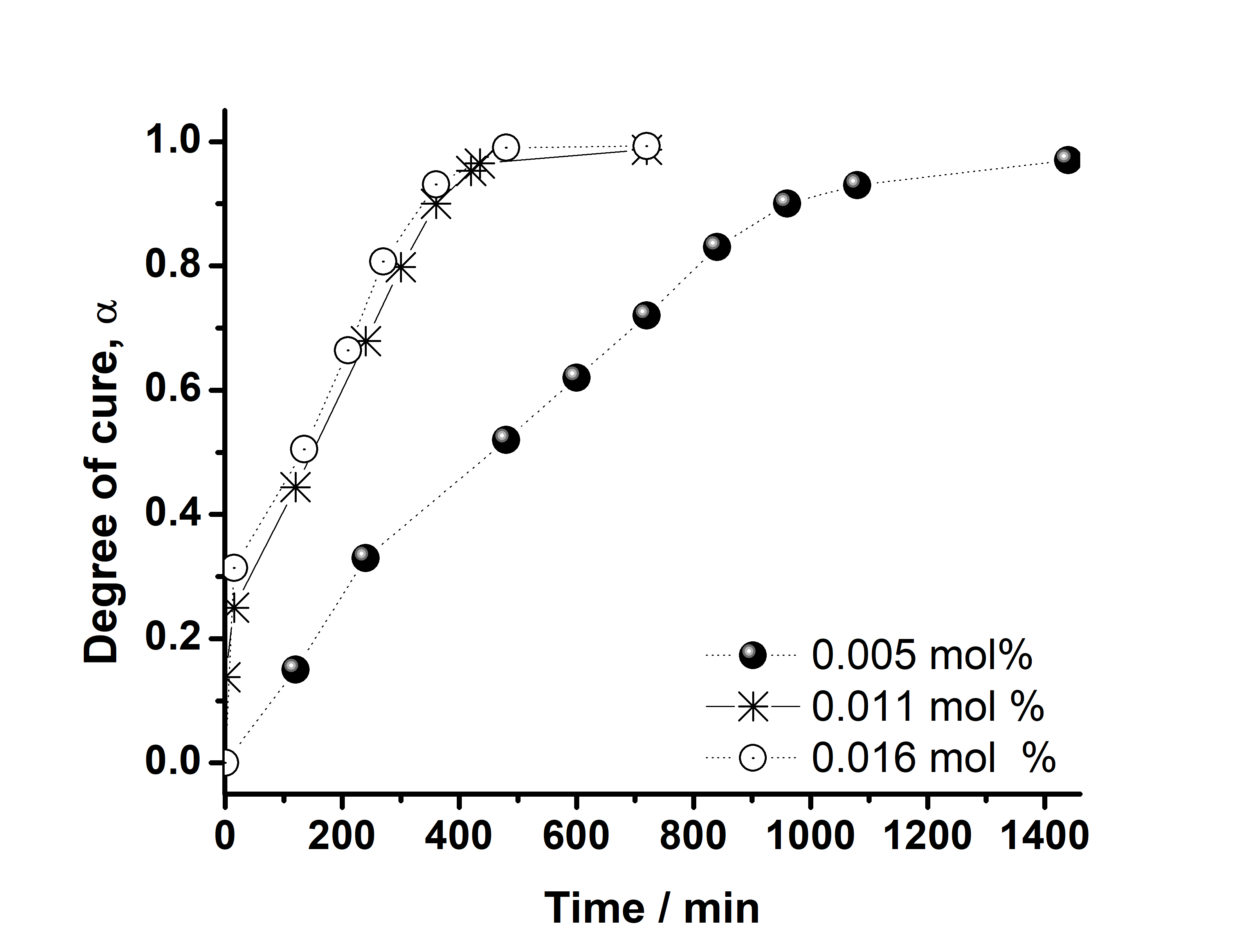 | Figure 7. Cure monitoring by in situ FT-IR at 25°C for ELO + 5.0 x 10-3 % mol of Al(OTf)3 |
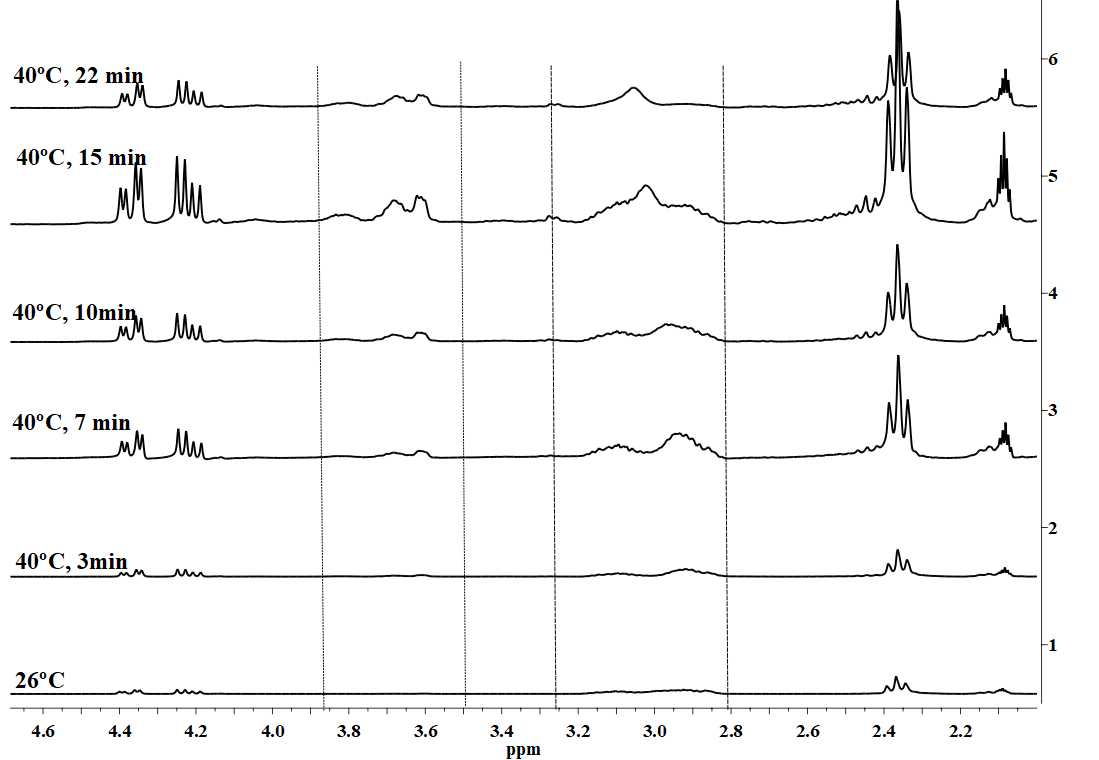 | Figure 8. Cure following by in situ 1H-NMR at 40°C for ELO + 36 x 10-3 % mol of Al(OTf)3 |
4. Conclusions
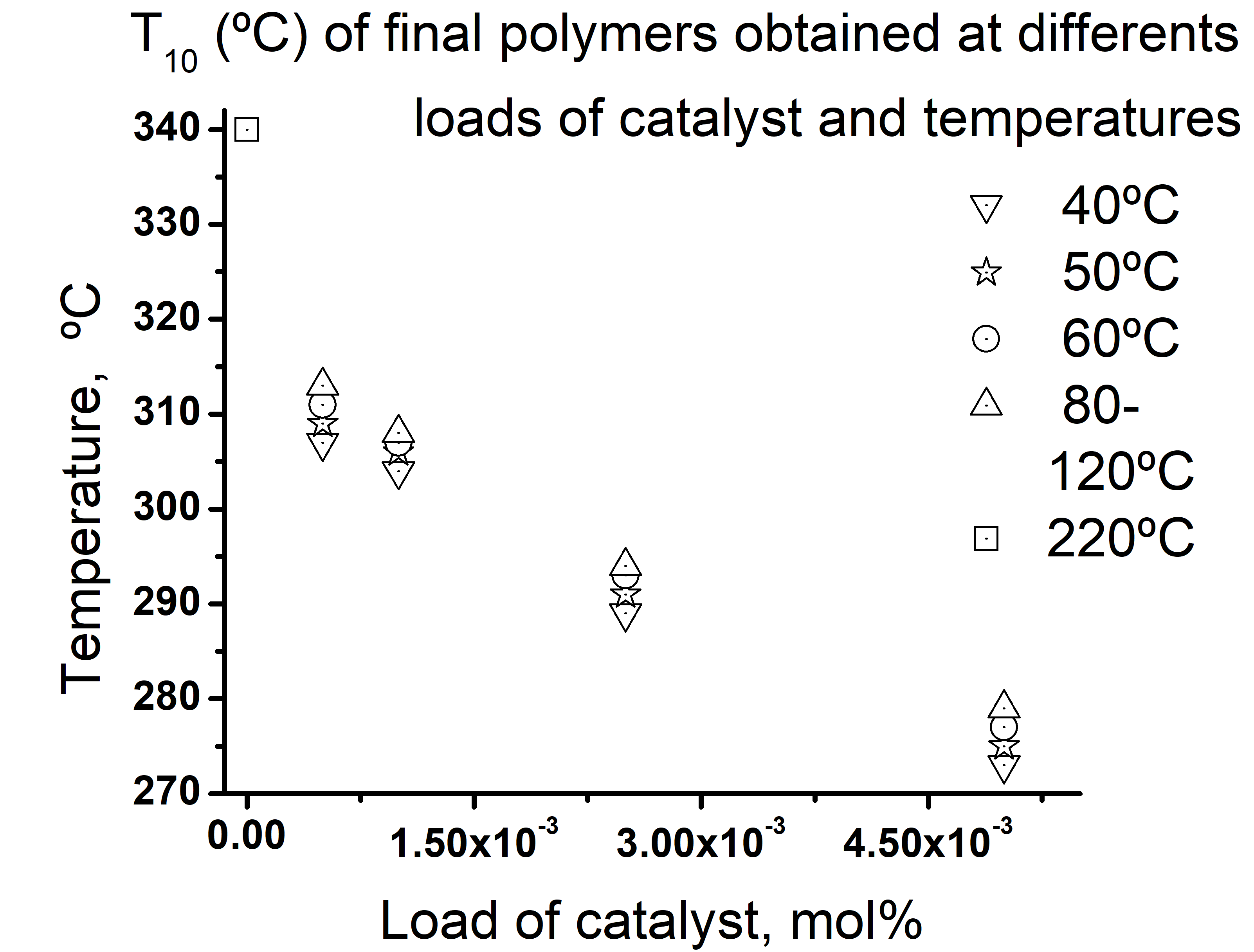
- The important of this thermal following is that aluminum triflate resulted in a very effective catalyst for directly polymerization of ELO from very low loads, 5x10-4 mol% to 5x10-3 mol% for a very controlled process through a very range of temperatures from 25 to 100-120°C very much below their exothermal curve and to the cure temperature of pure ELO. Also, the reaction time was variable according to the temperature and load catalyst, but they ranged from 7 min for temperatures near to the exothermal curve and many days for temperatures of 40 and 50°C. Also, the T10 was also affected (diminished) more by the catalyst load than by the cure temperature. The results here showed could be useful for study profoundly the kinetic of some particular system of ELO + Al(OTf)3 with or without the presence of some crosslinker agent. DSC and HART-FTIR spectroscopies used for following the cure process had a very good agreement in the determination of the percent of cure of the studied mixtures.
ACKNOWLEDGEMENTS
- Authors thank to the CONACyT for the fellowship and to the M.C. Nieves Zavala from CCIQS, UAEM-UNAM for her technical support for the in situ 1H-NMR analysis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML