-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Physical Chemistry
p-ISSN: 2167-7042 e-ISSN: 2167-7069
2018; 8(1): 13-25
doi:10.5923/j.pc.20180801.02

Densities, Excess and Partial Molar Volumes of (DFM + n-pentane or n-hexane or n-heptane or n-octane) Binary Mixtures at (T = 293.15, 298.15 and 303.15) K and Atmospheric Pressure
Wilfred Ddamba, Belcher Fulele, Misael Silas Nadiye -Tabbiruka
Department of Chemistry, University of Botswana, Gaborone, Botswana
Correspondence to: Wilfred Ddamba, Department of Chemistry, University of Botswana, Gaborone, Botswana.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Densities (ρ) of pure difuryl methane (DFM), n-pentane or n-hexane or n-heptane or n-octane and those of (DFM + n-pentane or n-hexane or n-heptane or n-octane) binary mixtures over the entire composition range, have been measured at (T = 293.15, 298.15 and 303.15) K and atmospheric pressure. Excess molar volumes 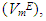 of each binary system were determined and correlated by the Redlich-Kister equation. The
of each binary system were determined and correlated by the Redlich-Kister equation. The 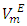 vs x2isothermsfor the (DFM + n-pentane or n-hexane or n-heptane) binary mixtures exhibit a sigmoidal behavior while that for the (DFM + n-octane) binary system positive deviation for
vs x2isothermsfor the (DFM + n-pentane or n-hexane or n-heptane) binary mixtures exhibit a sigmoidal behavior while that for the (DFM + n-octane) binary system positive deviation for 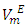 function was observed. The
function was observed. The 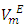 values decreased with temperature increase for each binary systems which suggested a possible structural effects. The
values decreased with temperature increase for each binary systems which suggested a possible structural effects. The 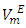 data have been used to derive excess partial molar volumes (
data have been used to derive excess partial molar volumes (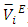 ). Results are discussed in terms of possible geometrical and dispersive intermolecular interactions.
). Results are discussed in terms of possible geometrical and dispersive intermolecular interactions.
Keywords: Difurylmethane, n-alkane, Excess molar volume, Excess partial molar volume, Binary mixtures, Intermolecular interactions
Cite this paper: Wilfred Ddamba, Belcher Fulele, Misael Silas Nadiye -Tabbiruka, Densities, Excess and Partial Molar Volumes of (DFM + n-pentane or n-hexane or n-heptane or n-octane) Binary Mixtures at (T = 293.15, 298.15 and 303.15) K and Atmospheric Pressure, Physical Chemistry, Vol. 8 No. 1, 2018, pp. 13-25. doi: 10.5923/j.pc.20180801.02.
Article Outline
1. Introduction
- Research activities in our laboratory consist of systematic measurements of physicochemical properties of non-aqueous binary mixtures in which difuryl methane (DFM) is one of the two components [1-4]. Studies of thermodynamic properties such as excess molar volumes contribute to understanding the nature of molecular interactions in liquid mixtures. It has been reported that DFM comprise of polar molecules and its liquid structure is determined by the dipole-dipole interactions [4].On the other hand for pure n-alkanes, the intermolecular interactions in part comprise of van der Waals forces. In addition, thermodynamic evidence obtained from Depolarized Raleigh Scattering studies on pure n-alkanes, indicates that there is short range orientational order between chains of molecules in the pure state which increases with the carbon chain length [5, 6]. The objective of the present study is to obtain information on intermolecular interactions in the binary mixtures of DFM with n-pentane or n-hexane or n-heptane or n-octane. We herewith report densities, ρ, excess molar volumes,
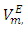 and excess partial molar volumes,
and excess partial molar volumes, 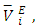 for (DFM + n-pentane or n-hexane or n-heptane or n-octane) binary mixtures at (T = 293.15, 298.15 and 303.15) K and atmospheric pressure. The
for (DFM + n-pentane or n-hexane or n-heptane or n-octane) binary mixtures at (T = 293.15, 298.15 and 303.15) K and atmospheric pressure. The 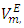 data of the binary mixtures have been fitted to the Redlich – Kister equation [7] to allow determination of the fitting parameters. The results obtained have been used to interpret intermolecular interactions that exist in these binary mixtures. A survey of the literature has indicated that there has been no reported study on volumetric properties for (DFM + n-pentane or n-hexane or n-heptane or n-octane) binary mixtures at (T = 293.15, 298.15 and 303.15) K.
data of the binary mixtures have been fitted to the Redlich – Kister equation [7] to allow determination of the fitting parameters. The results obtained have been used to interpret intermolecular interactions that exist in these binary mixtures. A survey of the literature has indicated that there has been no reported study on volumetric properties for (DFM + n-pentane or n-hexane or n-heptane or n-octane) binary mixtures at (T = 293.15, 298.15 and 303.15) K.2. Experimental
2.1. Materials and Purification of Solvents
- The following chemicals were used: n-pentane (Fluka, analytical reagent ≥ 99.8%), n-hexane (Merck Co., ≥ 98.0%), n- heptane (Merck Co., ≥ 99.0%), n-octane (Saarchem chemicals, 99.0%), furfuryl alcohol (Merck Co., 98.0%), borontrifluoride-dietherate, BF3(OEt2)2, (Sigma-Aldrich Chemicals Co., 99.0%). Each of (C5 - C8) n-alkanes was purified by distillation using 1m fractionation column fitted with glass beads. DFM was prepared as described elsewhere [8], and its purity was confirmed by 1H-NMR and density measurements. The elemental analysis of DFM was found to be as follows: Calcd for C9H8O2: C, 72.96; H, 5.44; O, 21.60. Found: C, 73.02; H, 5.46; O, 21.52%. The percentage carbon and hydrogen content of DFM were each slightly higher, while that for oxygen was lower than the expected values. This difference was attributed to a low contamination level of n-hexane solvent used in the DFM purification process.All purified organic liquids were stored in brown glass bottles and fractionally distilled just before use. The purity of each (C5 - C8) n-alkanes and DFM was tested by density measurement which was compared to literature values. Measured densities for pure components at various temperatures together with corresponding literature data are listed in Table 1. Ultrapure water required for the calibration of the densimeter was obtained as follows. Distilled water was first refluxed over KMnO4 and finally doubly distilled under nitrogen flow using a two-stage Heraeus-Destamat quartz still instrument. The conductivity of water was always less than 1.0x10-6 S-cm-1.
|
2.2. Apparatus and Procedure
- Each one of the (DFM + n-pentane or n-hexane or n-heptane or n-octane) binary mixtures was prepared by weighing appropriate amounts of DFM and the corresponding n-alkane co-solvent on an analytical balance (∆m = ±0.0001 g), by syringing each component into a stoppered flask. Pure components were first separately degassed in an ultrasonic bath shortly before sample preparations. All binary mixtures were completely miscible over the entire composition range. Solution density measurements were performed at atmospheric pressure with an Anton Paar DMA 4500 vibrating-tube densimeter at the experimental temperature. The densimeter was first calibrated with pure water and benzene as reference liquids. A sample volume of not more than 1.0 cm3 was needed to fill the densimeter cell and thermal equilibrium was attained quickly. The temperature of the sample was controlled electronically by means of a built-in thermostat (a semiconductor Peltier element and a resistance thermometer temperature control system) and was measured with an accuracy of ±0.01 K. The densimeter was calibrated after each set of four density measurements to offset possible instrument drift. A linear relation between the density of the fluid and the square of the vibrating period τ, (ρ = A + Bτ2), was assumed. Buoyancy corrections were made by taking into account of the air density at each of the three temperatures, the barometric pressure, and the relative humidity. Under such conditions triplet density measurements of each sample were reproducible to within ±0.01 kg-m-3. The reliability of density measurements was ascertained by comparing the experimental density of pure liquids with the corresponding literature values (Table 1) at the studied temperatures.
3. Results and Discussion
- Excess molar volumes
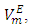 were calculated from density measurements for each of [DFM + n-pentane or n-hexane or n-heptane or n-octane) binary systems using equation (1) [13-15];
were calculated from density measurements for each of [DFM + n-pentane or n-hexane or n-heptane or n-octane) binary systems using equation (1) [13-15];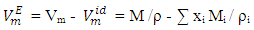 | (1) |
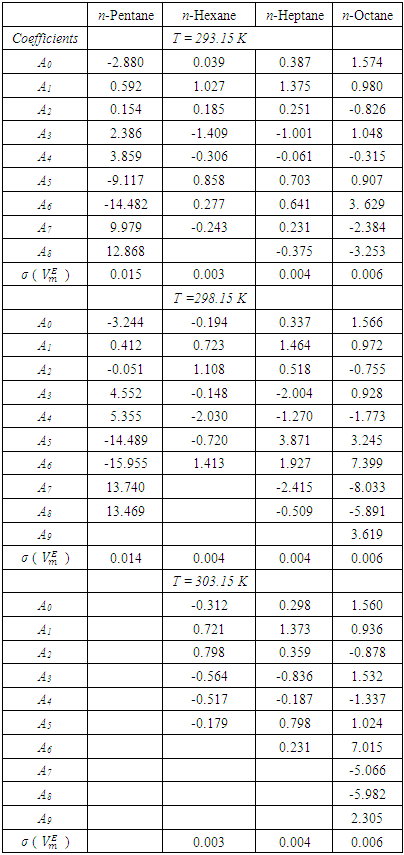
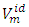 is the ideal molar volume, ρ is the density of the mixture, and xi, Mi and ρi are respectively the mole fraction, the molar mass and the density of the pure liquid component i. The Redlich-Kister polynomial equation (2) [7] in which all points are weighted equally was least-square fitted to the
is the ideal molar volume, ρ is the density of the mixture, and xi, Mi and ρi are respectively the mole fraction, the molar mass and the density of the pure liquid component i. The Redlich-Kister polynomial equation (2) [7] in which all points are weighted equally was least-square fitted to the 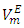 data (Table 2) for each binary system:
data (Table 2) for each binary system: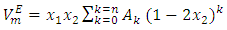 | (2) |
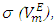 calculated from equation (3):
calculated from equation (3):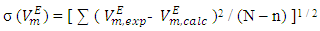 | (3) |
|
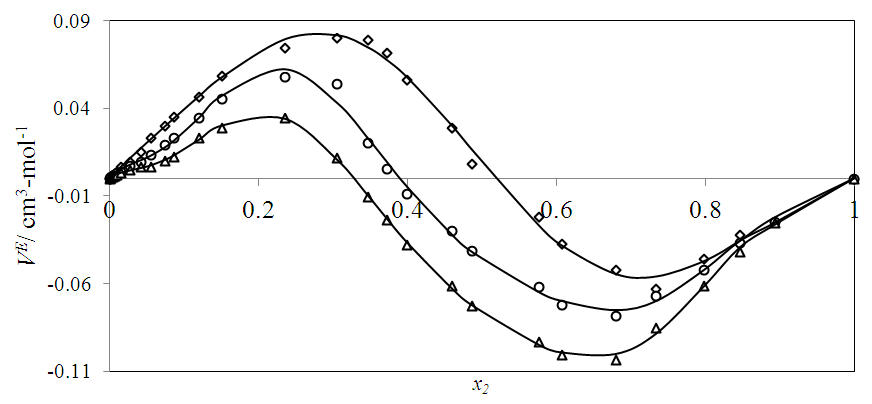
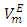 vs x2 data for each of the (DFM + n-pentane or n-hexane or n-heptane or n-octane) binary mixtures at 298.15 K. For each binary mixture, the fitted curve was calculated by using the corresponding Redlich-Kister polynomial regression coefficients (Table 3). Figure 1 shows that the
vs x2 data for each of the (DFM + n-pentane or n-hexane or n-heptane or n-octane) binary mixtures at 298.15 K. For each binary mixture, the fitted curve was calculated by using the corresponding Redlich-Kister polynomial regression coefficients (Table 3). Figure 1 shows that the 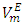 vs x2 isotherms for (DFM + n-pentane or n-hexane or n-heptane) binary mixtures exhibited sigmoidal-shaped behavior, with positive deviations limited to 0.00 ≤ x2 ≤ 0.039, 0.00 ≤ x2 ≤ 0.39 and 0.00 ≤ x2 ≤ 0.64 respectively, and negative deviations over the remaining x2 - ranges. It has been suggested that the sigmoidal-shaped behavior for the
vs x2 isotherms for (DFM + n-pentane or n-hexane or n-heptane) binary mixtures exhibited sigmoidal-shaped behavior, with positive deviations limited to 0.00 ≤ x2 ≤ 0.039, 0.00 ≤ x2 ≤ 0.39 and 0.00 ≤ x2 ≤ 0.64 respectively, and negative deviations over the remaining x2 - ranges. It has been suggested that the sigmoidal-shaped behavior for the 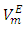 vs x2 isotherm arises from opposing effects which result from differences in energies of interaction between molecules in solution and packing effects [17, 18]. When DFM is added to the n-alkane, the mixing process may lead to a net destruction of the weak intermolecular interactions in the n-alkane and partial disruption of the dipole-dipole interactions in DFM liquid structure. In the low-x2 compositions, positive
vs x2 isotherm arises from opposing effects which result from differences in energies of interaction between molecules in solution and packing effects [17, 18]. When DFM is added to the n-alkane, the mixing process may lead to a net destruction of the weak intermolecular interactions in the n-alkane and partial disruption of the dipole-dipole interactions in DFM liquid structure. In the low-x2 compositions, positive 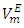 values indicate dominance of the entropy increasing dispersive intermolecular interactions due to possible loosening of the n-alkane molecular parking and the partial disruption of the dipole-dipole liquid structure in DFM which results in the observed volume expansion for each of the three binary systems. The sequence for the positive
values indicate dominance of the entropy increasing dispersive intermolecular interactions due to possible loosening of the n-alkane molecular parking and the partial disruption of the dipole-dipole liquid structure in DFM which results in the observed volume expansion for each of the three binary systems. The sequence for the positive 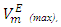 values is in the order: n-heptane > n-hexane > n-pentane and were: +0.188 cm3 mol-1 (at x2 = 0.254), +0.062 cm3 mol-1 (at x2 = 0.236) and+0.0474cm3 mol-1 (at x2 = 0.022), respectively. The inflection points for n-pentane, n-hexane and n-heptane occur at x2 = 0.039, x2 = 0.39 and x2 = 0.64 respectively. Thus, the DFM mole fraction at which the transition from a positive to negative
values is in the order: n-heptane > n-hexane > n-pentane and were: +0.188 cm3 mol-1 (at x2 = 0.254), +0.062 cm3 mol-1 (at x2 = 0.236) and+0.0474cm3 mol-1 (at x2 = 0.022), respectively. The inflection points for n-pentane, n-hexane and n-heptane occur at x2 = 0.039, x2 = 0.39 and x2 = 0.64 respectively. Thus, the DFM mole fraction at which the transition from a positive to negative 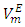 values occurs increases with the increase in the n-alkane chain length. Since weak van der Waals intermolecular interactions exist between n-alkane and DFM molecules, the negative
values occurs increases with the increase in the n-alkane chain length. Since weak van der Waals intermolecular interactions exist between n-alkane and DFM molecules, the negative 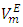 values observed for (DFM +n-pentane or n-hexane or n-heptane) binary mixtures may be arising from geometrical interstitial accommodation effects which result from the free volume differences between the unlike components in each the three binary mixtures [19]. The magnitude of
values observed for (DFM +n-pentane or n-hexane or n-heptane) binary mixtures may be arising from geometrical interstitial accommodation effects which result from the free volume differences between the unlike components in each the three binary mixtures [19]. The magnitude of 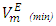 values is in the order: n-pentane > n-hexane > n-heptane and were -0.812 cm3 mol-1 (at x2 = 0.504), -0.075 cm3 mol-1 (at x2 = 0.680) and -0.039 cm3 mol-1 (at x2 = 0.848) respectively. It is possible that DFM component in each binary mixture, forms a relatively open liquid structure with sufficiently large cavities for the n-alkane molecular interstitial accommodation resulting in closer molecular packing which is reflected in negative
values is in the order: n-pentane > n-hexane > n-heptane and were -0.812 cm3 mol-1 (at x2 = 0.504), -0.075 cm3 mol-1 (at x2 = 0.680) and -0.039 cm3 mol-1 (at x2 = 0.848) respectively. It is possible that DFM component in each binary mixture, forms a relatively open liquid structure with sufficiently large cavities for the n-alkane molecular interstitial accommodation resulting in closer molecular packing which is reflected in negative 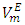 values for n-pentane or n-hexane or n-heptane in the x2 - range: 0.039 ≤ x2 ≤ 1.00 , 0.39 ≤ x2 ≤ 1.00 and 0.64 ≤ x2 ≤ 1.00 respectively. The trend in the
values for n-pentane or n-hexane or n-heptane in the x2 - range: 0.039 ≤ x2 ≤ 1.00 , 0.39 ≤ x2 ≤ 1.00 and 0.64 ≤ x2 ≤ 1.00 respectively. The trend in the 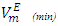 values suggest that the geometrical interstitial accommodation of the n-alkane molecules into the DFM liquid structure becomes less efficient as the n-alkane chain length increases. It can also be noticed that the (DFM + n-pentane) binary system exhibits a comparatively large
values suggest that the geometrical interstitial accommodation of the n-alkane molecules into the DFM liquid structure becomes less efficient as the n-alkane chain length increases. It can also be noticed that the (DFM + n-pentane) binary system exhibits a comparatively large 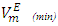 in comparison to the (DFM + n-hexane n-heptane) binary systems. The contributing factors to this behavior is the difference in molecular shape and size between DFM and n-pentane or n-hexane n-heptane. DFM being a polar molecule, would tend to remain self-associated in the binary solution. It is possible that the smaller n-pentane molecules are more efficiently accommodated into voids of DFM liquid structure in comparison with either n-hexane or n-heptane molecules causing more negative
in comparison to the (DFM + n-hexane n-heptane) binary systems. The contributing factors to this behavior is the difference in molecular shape and size between DFM and n-pentane or n-hexane n-heptane. DFM being a polar molecule, would tend to remain self-associated in the binary solution. It is possible that the smaller n-pentane molecules are more efficiently accommodated into voids of DFM liquid structure in comparison with either n-hexane or n-heptane molecules causing more negative 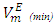 values for (DFM + n-pentane) binary system at each temperature investigated. For the (DFM + n-octane) binary system, positive
values for (DFM + n-pentane) binary system at each temperature investigated. For the (DFM + n-octane) binary system, positive 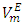 values were observed over the entire x2 range suggesting the dominance of the dispersive intermolecular interactions over the geometrical effects.
values were observed over the entire x2 range suggesting the dominance of the dispersive intermolecular interactions over the geometrical effects. 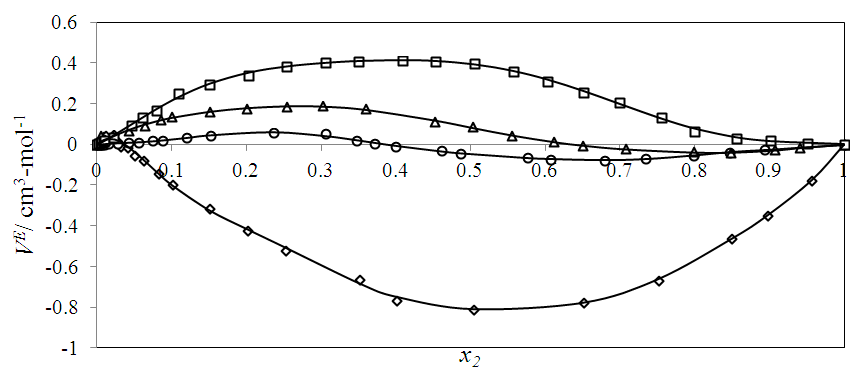 | Figure 1. Excess molar volume, 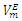 vs x2 for the [DFM (2) + n-alkane (1)] binary mixtures: (◊) n- pentane; (○) n-hexane; (∆) n-heptane and (□) n-octane at 298.15 K. The solid lines are from the appropriate Redlich-Kister fitting equations vs x2 for the [DFM (2) + n-alkane (1)] binary mixtures: (◊) n- pentane; (○) n-hexane; (∆) n-heptane and (□) n-octane at 298.15 K. The solid lines are from the appropriate Redlich-Kister fitting equations |
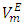 in general are of interest for better understanding of the structural behavior between molecules of components in a binary mixture. Figures 2, 3, 4 and 5 represent the temperature effect on
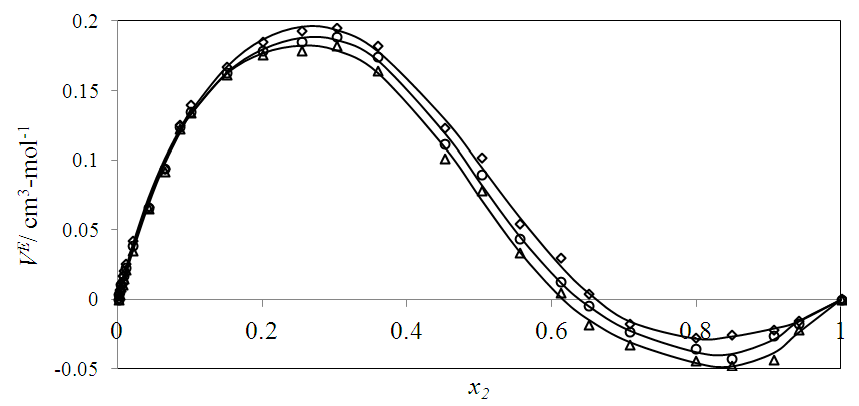
in general are of interest for better understanding of the structural behavior between molecules of components in a binary mixture. Figures 2, 3, 4 and 5 represent the temperature effect on 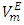 vs x2 isotherms for each of (DFM + n-pentane or n-hexane or n-heptane or n-octane) binary systems. It was observed that for each of the four binary systems, the algebraic magnitude of the
vs x2 isotherms for each of (DFM + n-pentane or n-hexane or n-heptane or n-octane) binary systems. It was observed that for each of the four binary systems, the algebraic magnitude of the 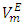 function over the entire composition range decreases with temperature rise. It was also observed that as temperature is raised, the
function over the entire composition range decreases with temperature rise. It was also observed that as temperature is raised, the 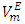 vs x2 isotherm for each of (DFM + n-pentane or n-hexane or n-heptane) systems maintains the sigmoidal behavior, but exhibited a diminished region for positive
vs x2 isotherm for each of (DFM + n-pentane or n-hexane or n-heptane) systems maintains the sigmoidal behavior, but exhibited a diminished region for positive 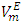 values and an enhanced region for negative
values and an enhanced region for negative 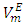 values. In general the increase in temperature would promote increased molecular motion which would result in a decrease in close molecular packing leading to a positive increase in
values. In general the increase in temperature would promote increased molecular motion which would result in a decrease in close molecular packing leading to a positive increase in 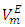 values. The experimentally observed volume contraction in each case may suggest that the volume expansion caused by temperature increase, results in a more favourable geometrical interstitial accommodation of n-alkane molecules into the expanded cavities in the DFM liquid structures. Similar temperature effect on binary systems containing n-alkanes has been reported elsewhere [12].
values. The experimentally observed volume contraction in each case may suggest that the volume expansion caused by temperature increase, results in a more favourable geometrical interstitial accommodation of n-alkane molecules into the expanded cavities in the DFM liquid structures. Similar temperature effect on binary systems containing n-alkanes has been reported elsewhere [12].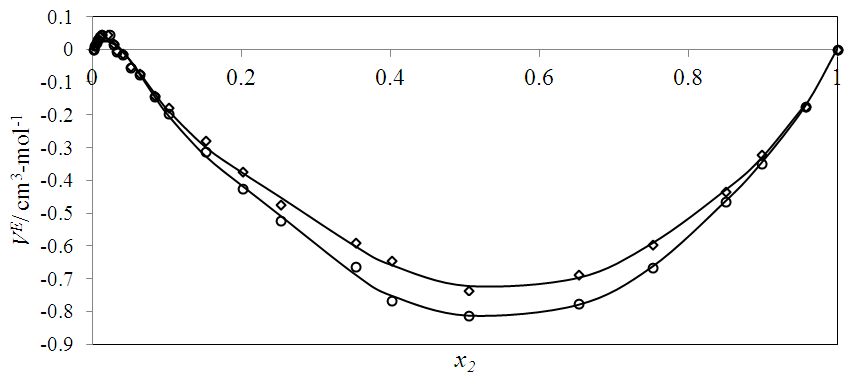 | Figure 2. Excess molar volume, 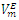 vs x2 for the [DFM (2) + n-pentane (1)] binary mixtures at (◊) 293.15 K; (○) 298.15 K. The solid lines are from the appropriate Redlich-Kister fitting equations vs x2 for the [DFM (2) + n-pentane (1)] binary mixtures at (◊) 293.15 K; (○) 298.15 K. The solid lines are from the appropriate Redlich-Kister fitting equations |
 | Figure 3. Excess molar volume, 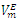 vs x2 for the [DFM (2) + n -hexane (1)] binary mixtures at (◊) 293.15 K; (○) 298.15 K, and (∆) 303.15 K. The solid lines are from the appropriate Redlich-Kister fitting equations vs x2 for the [DFM (2) + n -hexane (1)] binary mixtures at (◊) 293.15 K; (○) 298.15 K, and (∆) 303.15 K. The solid lines are from the appropriate Redlich-Kister fitting equations |
 | Figure 4. Excess molar volume, 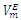 vs x2 for the [DFM (2) + n -heptane (1)] binary mixtures at (◊) 293.15 K; (○) 298.15 K, and (∆) 303.15 K. The solid lines are from the appropriate Redlich-Kister fitting equations vs x2 for the [DFM (2) + n -heptane (1)] binary mixtures at (◊) 293.15 K; (○) 298.15 K, and (∆) 303.15 K. The solid lines are from the appropriate Redlich-Kister fitting equations |
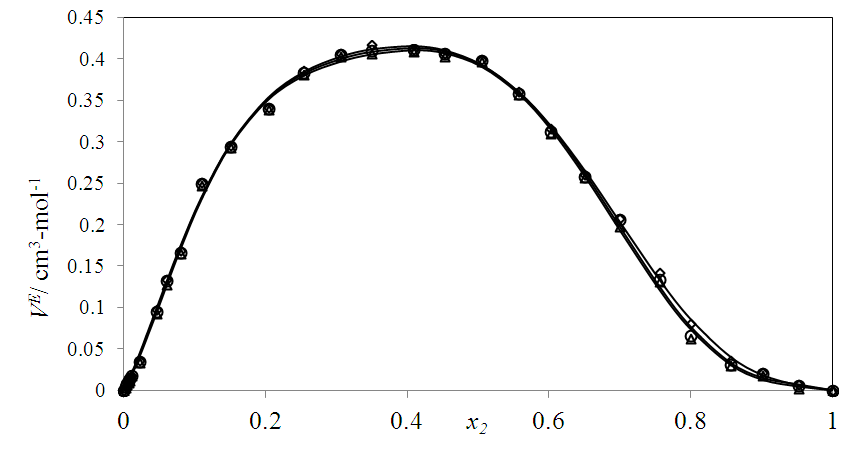 | Figure 5. Excess molar volume, 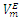 vs x2 for the [DFM (2) + n-octane (1)] binary mixtures at (◊) 293.15 K; (○) 298.15 K, and (∆) 303.15 K. The solid lines are from the appropriate Redlich-Kister fitting equations vs x2 for the [DFM (2) + n-octane (1)] binary mixtures at (◊) 293.15 K; (○) 298.15 K, and (∆) 303.15 K. The solid lines are from the appropriate Redlich-Kister fitting equations |
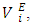 are more sensitive to changes in the aggregation schemes arising from the mixing process. The
are more sensitive to changes in the aggregation schemes arising from the mixing process. The 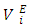 values of component i in a mixture describe the rate of change of the excess molar volume with composition, and represent the individual component response to the overall intermolecular interactions. Excess partial molar volumes for components in each binary system have been calculated in accordance with Equations (4) and (5) [14, 20];
values of component i in a mixture describe the rate of change of the excess molar volume with composition, and represent the individual component response to the overall intermolecular interactions. Excess partial molar volumes for components in each binary system have been calculated in accordance with Equations (4) and (5) [14, 20];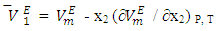 | (4) |
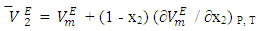 | (5) |
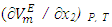 over the entire concentration range. Table 2 shows the calculated excess partial molar volumes,
over the entire concentration range. Table 2 shows the calculated excess partial molar volumes, 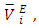 for components in each of the four binary systems at (T = 293.15, 298.15 and 303.15) K. Figures 6 (a) and 6 (b) depict the excess partial molar volumes of the components in each of (DFM + n-pentane or n-hexane or n-heptane or n-octane) binary systems at 298.15K. The
for components in each of the four binary systems at (T = 293.15, 298.15 and 303.15) K. Figures 6 (a) and 6 (b) depict the excess partial molar volumes of the components in each of (DFM + n-pentane or n-hexane or n-heptane or n-octane) binary systems at 298.15K. The 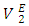 vs x2 isotherm for (DFM + n-pentane) binary system in the n-pentane rich region (0.00 < x2 < 0.017) (Figure 6 b) is characterized by a steep positive variation in
vs x2 isotherm for (DFM + n-pentane) binary system in the n-pentane rich region (0.00 < x2 < 0.017) (Figure 6 b) is characterized by a steep positive variation in 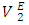 values, which may have resulted from the predominance of dispersive intermolecular interactions over the geometrical effects in this region. Thereafter the
values, which may have resulted from the predominance of dispersive intermolecular interactions over the geometrical effects in this region. Thereafter the 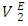 values are negative in the mole fraction range; (0.017 < x2 < 1.00), with a sharp minimum centred at x2 ≈ 0.06
values are negative in the mole fraction range; (0.017 < x2 < 1.00), with a sharp minimum centred at x2 ≈ 0.06 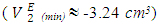 and broadened minimum at x2 ≈ 0.25
and broadened minimum at x2 ≈ 0.25 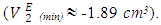 It is also observed that the variation with composition of
It is also observed that the variation with composition of 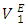 values for n-pentane (Figure 6 a) in this system revealed a small positive in the mole fraction range: 0.00 < x2 < 0.02 which may be due to dispersive interactions. Negative
values for n-pentane (Figure 6 a) in this system revealed a small positive in the mole fraction range: 0.00 < x2 < 0.02 which may be due to dispersive interactions. Negative 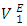 values for composition in the mole fraction range: (0.02 < x2 < 1.00) were observed for this system. Negative values for
values for composition in the mole fraction range: (0.02 < x2 < 1.00) were observed for this system. Negative values for 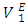 and
and 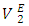 may be a result of geometrical effects in which the small n-pentane molecules are interstitially accommodated into the DFM liquid structure. The sharp negative variation of
may be a result of geometrical effects in which the small n-pentane molecules are interstitially accommodated into the DFM liquid structure. The sharp negative variation of 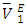 values in the DFM-rich region (low n-pentane concentrations) suggested enhanced structural effects when the n-pentane molecules are completely dispersed into the DFM liquid structure. The
values in the DFM-rich region (low n-pentane concentrations) suggested enhanced structural effects when the n-pentane molecules are completely dispersed into the DFM liquid structure. The 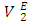 isotherms for (DFM + n-hexane or n-heptane or n-octane) systems exhibit sigmoidal behaviour (Figure 6 b), in which the positive magnitude of
isotherms for (DFM + n-hexane or n-heptane or n-octane) systems exhibit sigmoidal behaviour (Figure 6 b), in which the positive magnitude of 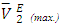 values increase with the n-alkane chain length in the order: n-octane > n-heptane > n-hexane. The
values increase with the n-alkane chain length in the order: n-octane > n-heptane > n-hexane. The 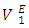 values for n-alkane components in each of (DFM + n-hexane or n-heptane or n-octane) systems exhibited a similar trend to
values for n-alkane components in each of (DFM + n-hexane or n-heptane or n-octane) systems exhibited a similar trend to 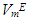 or
or 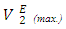 at a specified temperature. Thus at each temperature investigated, the magnitude of
at a specified temperature. Thus at each temperature investigated, the magnitude of 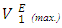 values was in the order: n-hexane < n-heptane < n-octane respectively. The increase in the positive magnitude for
values was in the order: n-hexane < n-heptane < n-octane respectively. The increase in the positive magnitude for 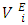 is further evidence of the increase in dominance of dispersive intermolecular interactions with increase of n-alkane chain-length. Figures 7 – 10 represent the effect of temperature on
is further evidence of the increase in dominance of dispersive intermolecular interactions with increase of n-alkane chain-length. Figures 7 – 10 represent the effect of temperature on 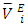 data for the two components in each of (DFM + n-pentane or n-hexane or n-heptane or n-octane) binary systems, at 293.15 and 303.15K. The positive magnitude of
data for the two components in each of (DFM + n-pentane or n-hexane or n-heptane or n-octane) binary systems, at 293.15 and 303.15K. The positive magnitude of 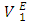 and
and 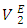 data in each binary system decreased with temperature rise. It was observed that the magnitude of the contraction in
data in each binary system decreased with temperature rise. It was observed that the magnitude of the contraction in 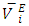 decreased with the n-alkane chain-length for the same temperature increase. The decrease in magnitude of
decreased with the n-alkane chain-length for the same temperature increase. The decrease in magnitude of 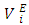 values with temperature rise corroborated the corresponding
values with temperature rise corroborated the corresponding 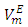 vs x2 isotherms (Figures 2-5) and suggested an increase in effectiveness of the geometrical interstitial accommodation effects with temperature rise.
vs x2 isotherms (Figures 2-5) and suggested an increase in effectiveness of the geometrical interstitial accommodation effects with temperature rise.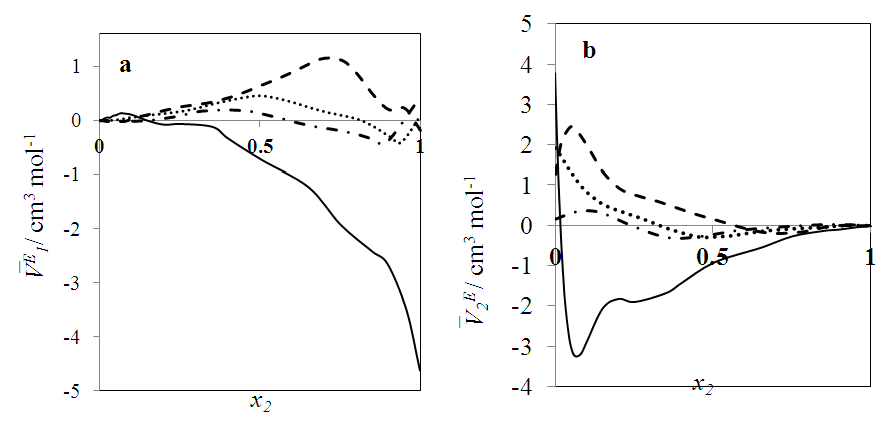 | Figure 6. Excess partial molar volumes,  vs x2 for the [DFM (2) + n-alkane (1)] binary mixtures: ( ) n-pentane; ( vs x2 for the [DFM (2) + n-alkane (1)] binary mixtures: ( ) n-pentane; ( ) n-hexane; ( ) n-hexane; ( ) n-heptane; ( ) n-heptane; ( ) n-octane; at 298.15 K ) n-octane; at 298.15 K |
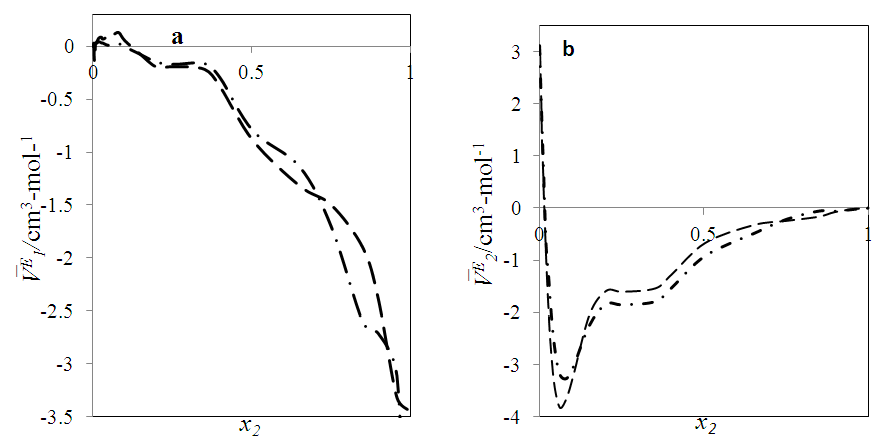 | Figure 7. Excess partial molar volume,  vs x2 for the [DFM (2) + n-pentane (1)] binary mixtures at temperatures: ( vs x2 for the [DFM (2) + n-pentane (1)] binary mixtures at temperatures: ( ) 293.15; ( ) 293.15; ( ) 298.15 K ) 298.15 K |
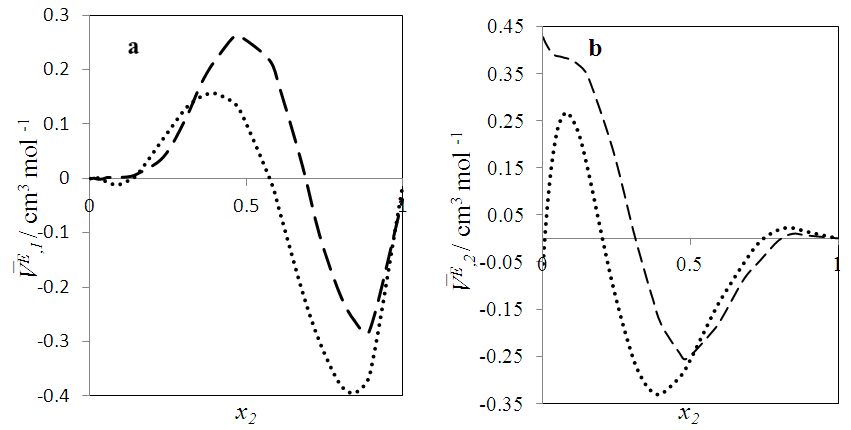 | Figure 8. Excess partial molar volume,  vs x2 for the [DFM (2) + n-hexane (1)] binary mixtures at temperatures: ( vs x2 for the [DFM (2) + n-hexane (1)] binary mixtures at temperatures: (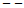 ) 293.15 and ( ) 293.15 and ( ) 303.15K ) 303.15K |
 | Figure 9. Excess partial molar volume, 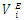 vs x2 for the [DFM (2) + n-heptane (1)] binary mixtures at temperatures: ( vs x2 for the [DFM (2) + n-heptane (1)] binary mixtures at temperatures: ( ) 293.15 and ( ) 293.15 and ( ) 303.15K ) 303.15K |
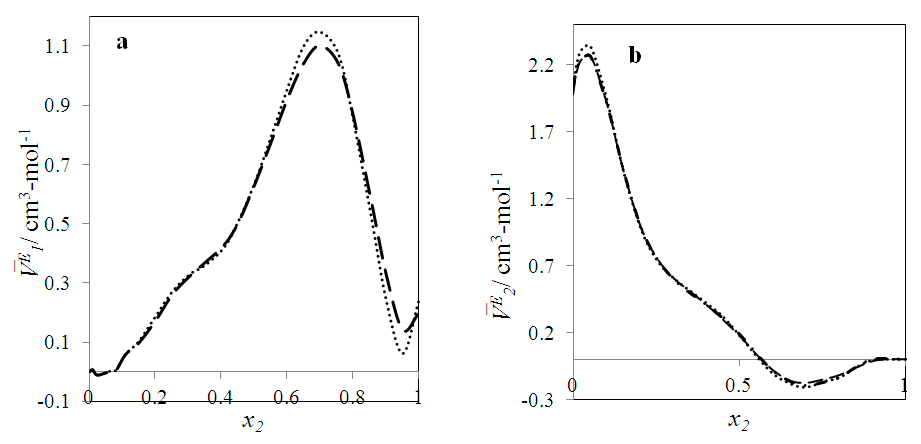 | Figure 10. Excess partial molar volume, 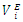 vs x2 for the [DFM (2) + n-octane (1)] binary mixtures at temperatures: ( vs x2 for the [DFM (2) + n-octane (1)] binary mixtures at temperatures: ( ) 293.15 and ( ) 293.15 and ( ) 303.15K ) 303.15K |
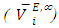 for components in the mixture [21, 22]. The optimized Redlich - Kister least squares fitting correlation coefficients for each (FM + n-pentane or n-hexane or n-heptane or n-octane) binary systems at the three temperatures investigated have been used to calculate values of the limiting excess partial molar volumes for n-alkanes
for components in the mixture [21, 22]. The optimized Redlich - Kister least squares fitting correlation coefficients for each (FM + n-pentane or n-hexane or n-heptane or n-octane) binary systems at the three temperatures investigated have been used to calculate values of the limiting excess partial molar volumes for n-alkanes 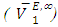 and
and 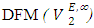 using equations (6) and (7) [21-23] respectively,
using equations (6) and (7) [21-23] respectively,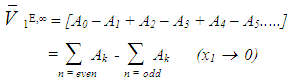 | (6) |
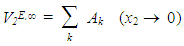 | (7) |
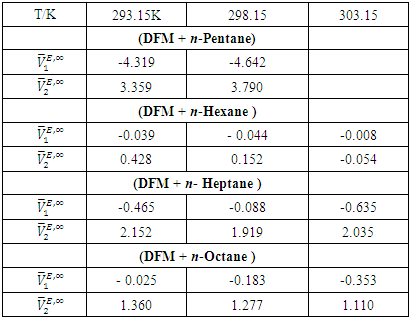
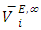 values are listed in Table 4. For all (DFM + n-pentane or n-hexane or n-heptane or n-octane) binary systems,
values are listed in Table 4. For all (DFM + n-pentane or n-hexane or n-heptane or n-octane) binary systems, 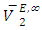 values were positive at the three temperatures investigated (293.15, 298.15 and 303.15) K, due to an expansion in volume. This suggests that in the low x2 region, the molar volume of DFM in the mixture is greater than its molar volume in the pure state. The observed positive
values were positive at the three temperatures investigated (293.15, 298.15 and 303.15) K, due to an expansion in volume. This suggests that in the low x2 region, the molar volume of DFM in the mixture is greater than its molar volume in the pure state. The observed positive 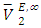 values for DFM in each binary system at the three temperatures investigated may be evidence for possible presence of DFM-DFM associate species in the infinite dilute solutions (low x2 region). The
values for DFM in each binary system at the three temperatures investigated may be evidence for possible presence of DFM-DFM associate species in the infinite dilute solutions (low x2 region). The 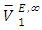 values for (C5-C8) n-alkane components in the binary mixtures were negative at the three temperatures investigated. Negative
values for (C5-C8) n-alkane components in the binary mixtures were negative at the three temperatures investigated. Negative 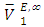 values in the high x2 range (low x1) suggest possible enhancement of the DFM-n-alkane molecular packing arising from the geometrical interstitial accommodation of n-alkane molecules into the bulk DFM-DFM, dipole-dipole liquid structure. The more negative
values in the high x2 range (low x1) suggest possible enhancement of the DFM-n-alkane molecular packing arising from the geometrical interstitial accommodation of n-alkane molecules into the bulk DFM-DFM, dipole-dipole liquid structure. The more negative 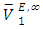 values observed for (DFM n-pentane) binary system at the two temperatures investigated suggested a efficient structural accommodation of n-pentane molecules into the DFM liquid structure.
values observed for (DFM n-pentane) binary system at the two temperatures investigated suggested a efficient structural accommodation of n-pentane molecules into the DFM liquid structure.
|
4. Conclusions
- In this study, measured densities on pure DFM, n-pentane, n-hexane, n-heptane and n-octane) as well as their binary mixtures, (DFM + n-pentane or n-hexane or n-heptane or n-octane), are reported at (T =293.15, 298.15 and 303.15) K. For each binary mixture, the density data were used to derive the excess molar volumes,
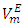 and excess partial molar volumes
and excess partial molar volumes 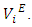 The
The 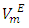 vs x2 isotherms for the (DFM + n-pentane or n-hexane or n-heptane) binary mixtures exhibit a sigmoidal behavior while for the (DFM + n-octane) binary system, positive deviation of the
vs x2 isotherms for the (DFM + n-pentane or n-hexane or n-heptane) binary mixtures exhibit a sigmoidal behavior while for the (DFM + n-octane) binary system, positive deviation of the 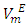 function was observed over the entire composition range. For each binary mixture, the magnitude of excess molar volume,
function was observed over the entire composition range. For each binary mixture, the magnitude of excess molar volume, 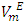 for mixture and
for mixture and 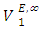 exhibited a decrease with rise in temperature. The decrease in the positive magnitude of
exhibited a decrease with rise in temperature. The decrease in the positive magnitude of 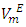 or
or 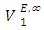 with increase in temperature was attributed to the enhancement of geometrical interstitial accommodation of n-alkane molecules into the expanded DFM liquid structure. The estimated excess partial molar volumes
with increase in temperature was attributed to the enhancement of geometrical interstitial accommodation of n-alkane molecules into the expanded DFM liquid structure. The estimated excess partial molar volumes 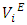 and limiting excess partial molar volumes
and limiting excess partial molar volumes 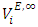 complimented the
complimented the 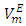 data for each of (DFM + n-pentane or n-hexane or n-heptane or n-octane) binary mixtures at each temperature investigated.
data for each of (DFM + n-pentane or n-hexane or n-heptane or n-octane) binary mixtures at each temperature investigated.ACKNOWLEDGEMENTS
- The financial support by the University of Botswana is gratefully acknowledged.
References
| [1] | Mokate, O., Ddamba, W., Volumetric properties of difurylmethane in methanol from 288.15 to 308.15K. Journal of Solution Chemistry, 34 (2005) 1327-1339. |
| [2] | Mokate, O., Ddamba, W Volumetric properties of (Difurylmethane + Alkan-1-ol) binary mixtures at 298.15K. Journal of Solution Chemistry, 35 (2006) 1493-1503. |
| [3] | Mokate, O., Ddamba, W., Effect of temperature on volumetric properties of [Difurylmethane + (C2-C6) Alkan-1-ol] binary systems: Analyses of new literature density data in the temperature range: 288.15 - 308.15K. Journal of Solution Chemistry, 37 (2008) 331-350. |
| [4] | Mmereki, B.T., Oathotse, I., Ddamba, W., Ultrasonic speeds and isentropic compressibilities of {Difurylmethane + (C1-C6) Alkan-1-ol} binary mixtures at T = 298.15K. Journal of Chemical Thermodynamics, 42 (2010) 1346-1351. |
| [5] | Patterson, D., Structure and the thermodynamics of non-electrolyte mixtures. Thermochimicta Acta, 267 (1995) 15-27. |
| [6] | Tancrede, P., Patterson, D., Lam, V, Thermodynamic effects of orientation order in chain-moecule mixtures. Part 3-Heats of mixing of dimethylsiloxanes with normal and branched alkanes. Journal of Chemical Society. Faraday Translations, 71 (1975) 985-996. |
| [7] | Redlich, O. and Kister, T. A., Algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 40, 345-348 (1948). |
| [8] | Buchwalter, S. L., The polymerization of furfuryl acetate in acetonitrile. J. Polymer Sci. 23 (1985) 2897-2911. |
| [9] | Weissberger, A., Organic Solvents: Physical Properties and Methods of Purification, New York, USA: John Wiley & Sons (1986) 55-57. Barbosa, E.F.G., Sousa, S.M.C, Soledade, M, Santos, M.S.C.S, Lampreia, I.M.S., Partial molar volumes of linear hydrocarbons in methanol in the very dilute region. Intermolecular interactions and H-bond effects. Physical Chemistry Chemical Physics, 3 (2001) 556-561. |
| [10] | Iloukhani, H., Sameti-Rezaei, M, Volumetric properties of methylcyclohexane with n- alkanes (C5-C10) at 293.15, 298.15,303.15K. Comparison with Prigogine-Flory-Patterson theory. Journal of Molecular Liquids, 126 (2006) 62-68. |
| [11] | Balán, J., Morávková, L., Linek, J, Excess Molar Volumes of the (Cyclohexane + Pentane, or Hexane, or Heptane, or Octane, or Nonane) Systems at the Temperature 298.15 K. Chemistry Paper, 61 (2007) 497-501. |
| [12] | Basu, M.A., Samanta, T., Das, D., Volumetric and acoustic properties of binary mixtures of tri-n-butyl phosphate with n-hexane, cyclohexane, and n-heptane from T = (298.15 to 323.15)K. Journal of Chemical Thermodynamics, 57 (2013) 335-343. |
| [13] | Davis, M.I., Thermodynamic and Related Studies of Amphiphile Plus Water-Systems. Chemical Society Reviews, 22 (1993) 127-134. |
| [14] | Douhẻret, G., Davis, M. I., Measurement, analysis, and utility of excess molar -(∂V/∂p). Chemical Society Reviews, 22 (1993) 43-50. |
| [15] | Blandamer, M.J., Equilibrium, frozen, excess and volumetric properties of dilute solutions. Chemical Society Reviews, 27 (1998) 73-79. |
| [16] | Miller, J.N., Miller, J.C., Statistics and chemometrics for analytical chemistry, Harlow, England, Ashford Color Press (2005) 256-257. |
| [17] | Treszczanowicz, A.J., Kiyohara, O., Benson, G. C., Excess volumes of n-alkanols + n-alkaness IV. Binary mixtures of decan-1-ol + n-pentane, + n-hexane, + n-octane, + n-decane, + n-hexadecane. Journal of Chemical Thermodynamics, 13 (1981) 253-260. |
| [18] | Lepori, L., Gianni, P., Spanedda, A., Matteoli, E., Thermodynamic study of (heptane + amine) mixtures. II. Excess and partial molar volumes at 298.15 K. Journal of Chemical Thermodynamics, 43 (2011) 805-813. |
| [19] | Basu, M.A., Samanta, T., Das, D., Volumetric and acoustic properties of binary mixtures of tri-n-butyl phosphate with n-hexane, cyclohexane, and n-heptane from T = (298.15 to 323.15)K. Journal of Chemical Thermodynamics, 57 (2013) 335-343. |
| [20] | Douhẻret, G., Davis, M. I., Hoiland, H, Speeds of sound and excess molar volumetric properties of mixtures of water with ethylene glycol monopropyl ether at 298.15 K. Journal of Molecular Liquids, 80 (1999) 1-18. |
| [21] | Acree Jr, W.E., Thermodynamic properties of nonelectrolytes, Orlando, Florida, USA: Academic Press (1984) 15-19. |
| [22] | Orge, B., Iglesias, M., Marino, G., Tojo, J., Thermodynamic properties of (acetone + methanol + 2-butanol) at T = 298.15 K. Journal of Chemical Thermodynamics, 31 (1999) 497-512. |
| [23] | Maham, Y., Teng, T. T., Hepler, L.G., Mather, A. E., Densities, excess molar volumes, and partial molar volumes for binary mixtures of water with monoethanolamine, diethanolamine, and triethanolamine from 25°C to 80°C. Journal of Solution Chemistry, 23 (1994) 195-205. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
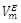 and excess partial molar volumes
and excess partial molar volumes 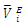 (cm3 mol-1) for (DFM + n-Pentane or n-Hexane or n-Heptane or n-Octane) binary mixtures at (T = 293.15, 298.15 and 303.15) K
(cm3 mol-1) for (DFM + n-Pentane or n-Hexane or n-Heptane or n-Octane) binary mixtures at (T = 293.15, 298.15 and 303.15) K
 for the components in [DFM + (C5-C8) n-alkane] binary systems at experimental temperatures
for the components in [DFM + (C5-C8) n-alkane] binary systems at experimental temperatures