-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Physical Chemistry
p-ISSN: 2167-7042 e-ISSN: 2167-7069
2018; 8(1): 1-12
doi:10.5923/j.pc.20180801.01

Role of Anion in the Electrochemical Dissolution of Copper and Its Inhibition by Diethyl Dithiocarbamate in Neutral Aqueous Solutions
B. A. Abd-El-Nabey, A. Eldissouky, H. A. Fetouh, M. E. Mohamed
Faculty of Science, Chemistry Department, Alexandria University, Alexandria, Egypt
Correspondence to: B. A. Abd-El-Nabey, Faculty of Science, Chemistry Department, Alexandria University, Alexandria, Egypt.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Electrochemical dissolution of copper in 0.5M aqueous solution of NaNO3, Na2SO4, NaCl or NaI in absence and presence of different concentrations of diethyl dithiocarbamate (DEDTC) has been studied using potentiodynamic polarization, electrochemical impedance spectroscopy (EIS) and cyclic voltametry techniques. In absence of DEDTC, the results indicated that oxidation of copper greatly depends on the kind of anion of the electrolyte. In NaNO3 and Na2SO4 solutions, oxidation of copper takes place through a two-electron transfer step Cu  Cu(II), however in NaCl and NaI solutions, oxidation of copper occurs through two consecutive one-electron transfer steps Cu
Cu(II), however in NaCl and NaI solutions, oxidation of copper occurs through two consecutive one-electron transfer steps Cu  Cu(I) and Cu (I)
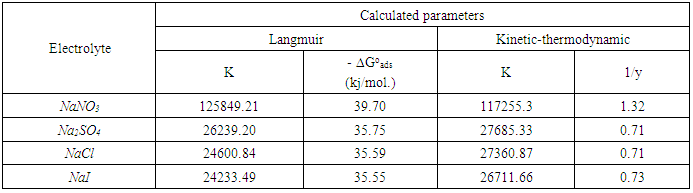
Cu(I) and Cu (I)  Cu(II). These results were discussed on the basis that the mechanism of oxidation of copper depends on the adsorbability of the anion of the electrolyte at the Cu/solution interface which affects each of the potential of zero charge (PZC) and the surface charge of the metal. In presence of DEDTC, the results indicated that its inhibition efficiency for corrosion of copper decreases in the electrolytes containing adsorbable anions due to the competitive adsorption between the anion of the electrolyte and that of DEDTC. Theoretical fitting of the Langmuir isotherm and the Kinetic-thermodynamic model for the adsorption of DEDTC on the copper surface in the four examined electrolytes are tested to clarify the nature of adsorption. The results showed that the value of the standard free energy change of adsorption (∆Goads) for the tested four electrolytes equal (-35.55 to -39.70 kj/mol.) indicating that the adsorption process of DEDTC on the copper surface is comprehensive (chemical and physical adsorption).
Cu(II). These results were discussed on the basis that the mechanism of oxidation of copper depends on the adsorbability of the anion of the electrolyte at the Cu/solution interface which affects each of the potential of zero charge (PZC) and the surface charge of the metal. In presence of DEDTC, the results indicated that its inhibition efficiency for corrosion of copper decreases in the electrolytes containing adsorbable anions due to the competitive adsorption between the anion of the electrolyte and that of DEDTC. Theoretical fitting of the Langmuir isotherm and the Kinetic-thermodynamic model for the adsorption of DEDTC on the copper surface in the four examined electrolytes are tested to clarify the nature of adsorption. The results showed that the value of the standard free energy change of adsorption (∆Goads) for the tested four electrolytes equal (-35.55 to -39.70 kj/mol.) indicating that the adsorption process of DEDTC on the copper surface is comprehensive (chemical and physical adsorption).
Keywords: Copper, Corrosion, Inhibitors, Diethyl Dithiocarbamate
Cite this paper: B. A. Abd-El-Nabey, A. Eldissouky, H. A. Fetouh, M. E. Mohamed, Role of Anion in the Electrochemical Dissolution of Copper and Its Inhibition by Diethyl Dithiocarbamate in Neutral Aqueous Solutions, Physical Chemistry, Vol. 8 No. 1, 2018, pp. 1-12. doi: 10.5923/j.pc.20180801.01.
Article Outline
1. Introduction
- Copper and its alloys have a wide range of applications in industry and seawater systems, such as shipbuilding, heat exchange systems, water pipelines and seawater desalination owing to the excellent properties such as mechanical workability, thermal and electrical conductivity. However copper and its alloys have good resistance, they suffer different forms of corrosion depending on the service environment, particularly in the presence of chloride ions. Therefore, the protection of copper and its alloys against corrosion has attracted the attention of many scientists [1-7].The use of organic inhibitors is one of the most efficient approaches for protecting copper against corrosion [8-13]. There are numerous reports points out the importance of heteroatoms such as N, O, S and P in organic compounds to give good corrosion inhibition of copper. The efficiency ofsulfur-containing compounds for corrosion inhibition of copper in a variety of media has been reported [14-17]. Benzotriazole is one of the most efficient organic inhibitors for the corrosion of copper in neutral-chloride media [18]. In addition, amines, azoles derivatives and mercapto-organic compounds are also potentially effective copper corrosion inhibitors [19-24]. Dithiocarbamates were shown as good inhibitors for the corrosion of copper in neutral chloride media; its inhibition was attributed to the formation of an insoluble Cu (II)-chelate film [25-27]. Adsorption of ammonium pyrolidine dithiocarbamate (PDTC) on copper surface was investigated by potentiodynamic polarization, electrochemical impedance spectroscopy measurements and surface analysis. The electrochemical results indicated that PDTC adsorbs rabidly on copper surface and act as mixed type inhibitor. Surface analysis confirmed the adsorption of PDTC on copper and the formation of Cu-PDTC complexes through the sulfur atom. XPS results showed that PDTC bonds with cuprous species indicating the formation of Cu(I)-PDTC complex.Dithiocarbamate compounds has been considered as fungicides which have low or moderate toxicity i.e it is not have acute hazard [28, 29]. Also, dithiocarbamate extensively used as agriculture biocide (pesticide), used before planting for elimination of soil pathogens, weeds and nematodes and used after planting as herbicide [30].It is well known that the electrochemical dissolution of metals in aqueous solutions is greatly affected by the kind of ions of the electrolyte. In our previous recent work [31] we studied the co-operative effect of chloride ions and some natural extract in retarding corrosion of steel in neutral media. Effect of lupine and damsisa extracts on the corrosion of steel in 0.5M Na2SO4 solution free from and containing 0.01M or 0.1M NaCl were examined by potentiodynamic and EIS techniques. Increasing chloride ion concentration in the solution led to increase of the inhibition efficiency of the extracts and this behavior was explained on the basis of co-operative mechanism of adsorption.In this work we studied the electrochemical dissolution of copper in 0.5M solutions of NaNO3, Na2SO4, NaCl and NaI in absence and presence of different concentrations of sodium diethyl dithiocarbamate in order to explore the role of the anion of the electrolyte in the mechanism of electrochemical dissolution of copper and its inhibition.
2. Experimental Methods
2.1. Chemicals and Materials
- An electrochemical measurement takes place in a three electrode cell with Copper as a working electrode that had the chemical composition Cu 99.5% and Ca 0.5%. A cylinder of copper was fixed in a rod of poly tetrafluoroethylene (PTFE) (Teflon) by an epoxy resin in such a way that only one side of constant surface area (0.2827 cm2) left uncovered and in contact with the solution. Copper wire was enclosed in a glass tube which acted as a holder as well as insulator. Graphite sheet was used as auxiliary electrode and saturated calomel electrode (SCE) as reference electrode. Before each experiment, the working electrode was polished with a series of emery papers of different grit sizes (120-1200). After polishing, the working electrode was washed with double distilled water and dried at room temperature. All the measurements were done under unstirred conditions at 30.0±0.2 oC in solutions open to the atmosphere. For each experiment 50 mL of electrolyte was used. All chemicals were purchased from Sigma-Aldrich Co.
2.2. Electrochemical Measurements
- The polished electrode cleaned as mentioned before and introduced in the electrochemical cell that was filled by 50 mL of the tested solution. The cell was thermostated for 20 minutes before starting the experiment. The open circuit potential of working electrode result from the formation of electrical double layer at metal/solution interface was followed as a function of time for 15 min, until steady state potential at which the variation of potential is 1.0 mV/ min. is established to ensure reliable measurements.Electrochemical impedance measurements were achieved by connecting the electrochemical cell to Gill AC impedance instrument. The Gill AC instrument was controlled by personal computer, for data logging and data analysis. The frequency range studied was 0.1 ≤ f ≤ 1.0 x104 Hz. Amplitude of 10.0 mV peak to peak was used for alternating current signal for all impedance spectroscopy measurements with respect the open circuit potential. Five data points were taken for each decade of frequency or for each change in frequency by ten multiplications. Gill AC serial number 604 sequencer software used for all the electrochemical measurements. The software Zsimpwin was used for analysis the impedance spectra of Nyquist plots using the suitable equivalent circuit model that well fit Nyquist plots with small error.Potentiodynamic polarization technique was used to determine cathodic and anodic polarization curves of copper in different tested aqueous solutions by sweeping the applied overpotentials starting with -250 mV (for cathodic polarization) reaching to rest potential (Erest) then continuing the anodic polarization by applying +250 mV above the rest potential. All electrode potentials were measured with respect to the saturated calomel electrode (SCE).
3. Results and Discussion
3.1. Effect of Anion on the Electrochemical Dissolution of Copper
- The mechanism of the electrochemical dissolution of copper in Cl- media has been extensively studied in the literature [25-27, 32-38], and proposed that copper dissolution involves first electronic transfer corresponds to the oxidation of copper to cuprous chloride species followed by a chemical step producing CuCl2-.
 | (1) |
 | (2) |
3.1.1. Potentiodynamic Polarization Results
- Figure 1 shows the polarization curves of copper in 0.5M solutions of NaNO3, Na2SO4, NaCl and NaI. The cathodic curve of copper in NaCl shows limiting current indicating that the reduction process is the diffusion controlled reduction of oxygen. However, the cathodic polarization curves in the other electrolytes show Tafel behavior and the reduction process is mainly the reduction of water. The anodic polarization curves of copper in each of NaNO3 and Na2SO4 show a characteristic Tafel behavior indicating that the oxidation of copper takes place through one step controlled by charge transfer. The anodic polarization curve of copper in NaCl shows an inflection at -0.125 V (vs. SCE), this inflection was reported previously in a recent work [39] and mainly corresponds to the second electron oxidation step of copper [Cu (I)
 Cu (II)]. The anodic polarization curve of copper in NaI shows a two steps oxidation process. Figure 1 shows also that the corrosion potential (Ecorr) of copper is greatly affected by kind of electrolyte and shifts to more negative values with increasing the adsorbability of the anion of the electrolyte from NO3- to I- in the order:NO3-> SO42- > Cl- > I-The above results can be discussed on the basis that adsorption of anions of the electrolyte at the Cu/solution interface creates a negative charge on the diffuse double layer of copper which enhance the uptake of the cuprous cation from the metal surface to the solution side. This is the case of the electrochemical dissolution of copper in NaCl and NaI solutions, where the oxidation takes place at more negative potentials and through two consecutive steps, Cu
Cu (II)]. The anodic polarization curve of copper in NaI shows a two steps oxidation process. Figure 1 shows also that the corrosion potential (Ecorr) of copper is greatly affected by kind of electrolyte and shifts to more negative values with increasing the adsorbability of the anion of the electrolyte from NO3- to I- in the order:NO3-> SO42- > Cl- > I-The above results can be discussed on the basis that adsorption of anions of the electrolyte at the Cu/solution interface creates a negative charge on the diffuse double layer of copper which enhance the uptake of the cuprous cation from the metal surface to the solution side. This is the case of the electrochemical dissolution of copper in NaCl and NaI solutions, where the oxidation takes place at more negative potentials and through two consecutive steps, Cu  Cu (I) and Cu (I)
Cu (I) and Cu (I)  Cu (II). However, in presence of the non-or weak adsorbable anions such as NO3- or SO42- the electrochemical dissolution of copper becomes more difficult and takes place at less negative potentials through a single two electron transfer step Cu
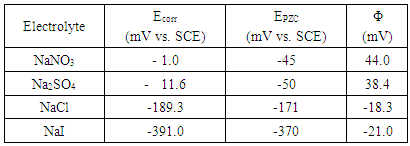
Cu (II). However, in presence of the non-or weak adsorbable anions such as NO3- or SO42- the electrochemical dissolution of copper becomes more difficult and takes place at less negative potentials through a single two electron transfer step Cu  Cu(II). To confirm this proposal the potential of zero charge (PZC) of copper in the four electrolytes has been measured and also the cyclic voltamogram has been investigated.
Cu(II). To confirm this proposal the potential of zero charge (PZC) of copper in the four electrolytes has been measured and also the cyclic voltamogram has been investigated.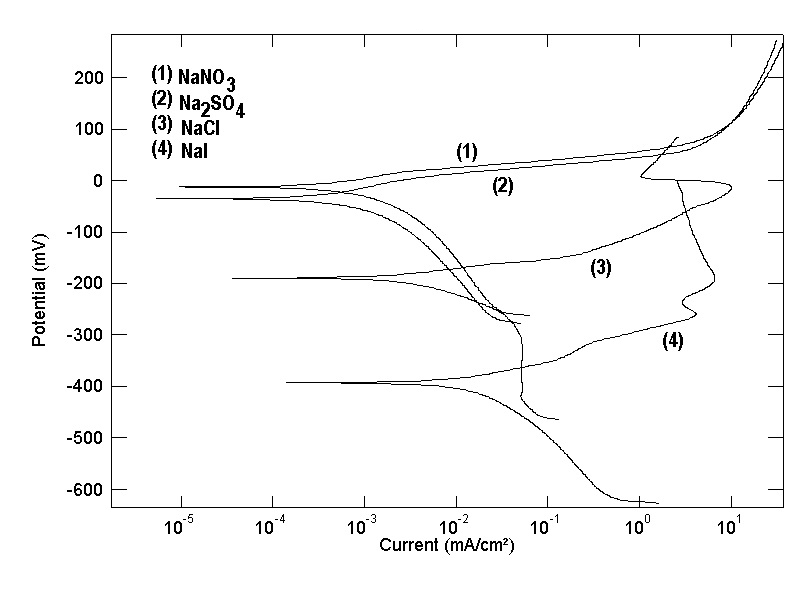 | Figure 1. Potentiodynamic polarization curves of copper in 0.5 M aqueous solution of NaNO3, Na2SO4, NaCl and NaI |
3.1.2. Potential of Zero Charge (EPZC) Results
- The potential of zero charge (EPZC) and the surface charge (Φ) of copper in 0.5M solutions of the electrolytes NaNO3, Na2SO4, NaCl and NaI were determined using the electrochemical impedance spectroscopy (EIS) technique. The impedance measurements were performed at certain cathodic and anodic overpotentials around Erest of copper and values of the charge transfer resistance (Rct) and electrical double layer capacity (Qdl) are estimated by fitting the Nyquist plots to the equivalent circuit in figure 2.
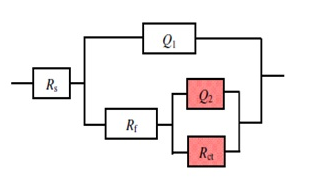 | Figure 2. The equivalent circuit model used to fit the EIS experiment |
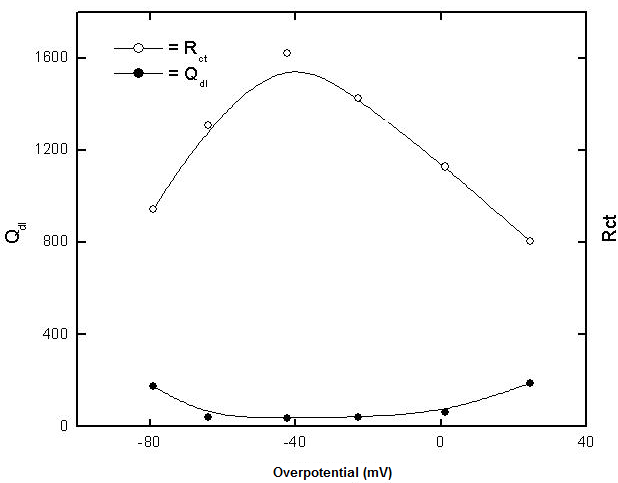 | Figure 3. Variation of each of Qdl and Rct with potential of copper in 0.5 M aqueous solution of NaNO3 |
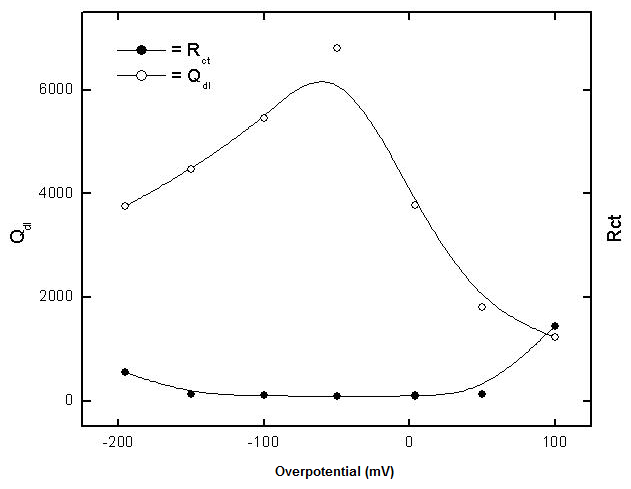 | Figure 4. Variation of each of Qdl and Rct with potential of copper in 0.5 M aqueous solution of Na2SO4 |
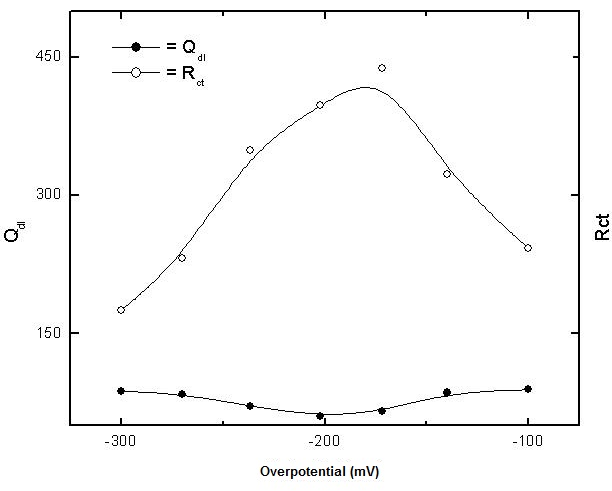 | Figure 5. Variation of each of Qdl and Rct with potential of copper in 0.5M aqueous solution of NaCl |
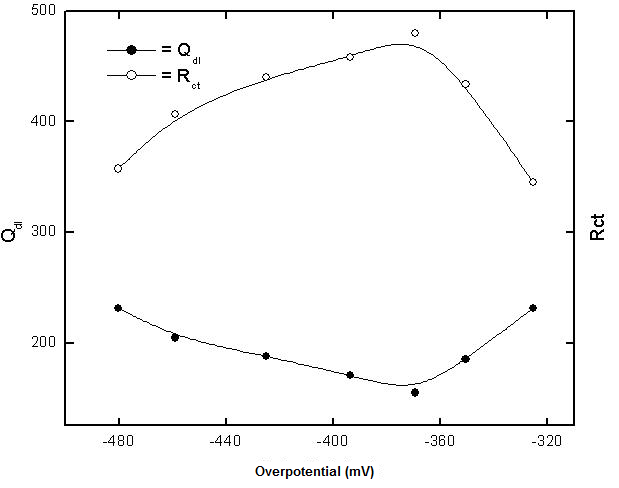 | Figure 6. Variation of each of Qdl and Rct with potential of copper in 0.5 M aqueous solution of NaI |
|
3.1.3. Cyclic Voltametric Results
- Several authors [26, 42, 43] studied the cyclic voltametry of copper in artificial seawater as well as in seawater. They reported that the cyclic voltamogram of copper contains two anodic peaks related to the copper oxidation to Cu (I) and Cu (II). Figures (7, 8) show the cyclic voltamograms of copper in the four studied electrolytes. It is clear that the cyclic voltamograms of copper in each of NaNO3 and Na2SO4 contain only one peak at 0.280 and at 0.270 volt (vs. SCE). However, the cyclic voltamogram of copper in each of NaCl and NaI contain two anodic peaks; for copper in NaCl the two peaks located at 0.110 and 0.250 while for copper in NaI the two peaks located at - 0.200 and 0.260 volt (vs. SCE). The above results show a good agreement with the potentiodynamic polarization and potential of zero charge results and give support to the proposal stating that the adsorption of the anions at copper/solution interface enhance the uptake of the cuprous ion making preoxidation of copper at more negative potential and the mechanism of electrochemical dissolution of copper goes through two consecutive one electron transfer steps. A further support to the above results is given in figure 9 which shows the cyclic voltamogram of copper in 0.5M Na2SO4+0.05M NaI. It can be seen that addition of the strong adsorbable I- ion to SO42- anions leads to the appearance of two anodic peaks at -0.25 and 0.10 volt (vs. SCE) in the cyclic voltamogram of copper in addition to the original peak in presence of Na2SO4 only at 0.270 volt (vs. SCE).
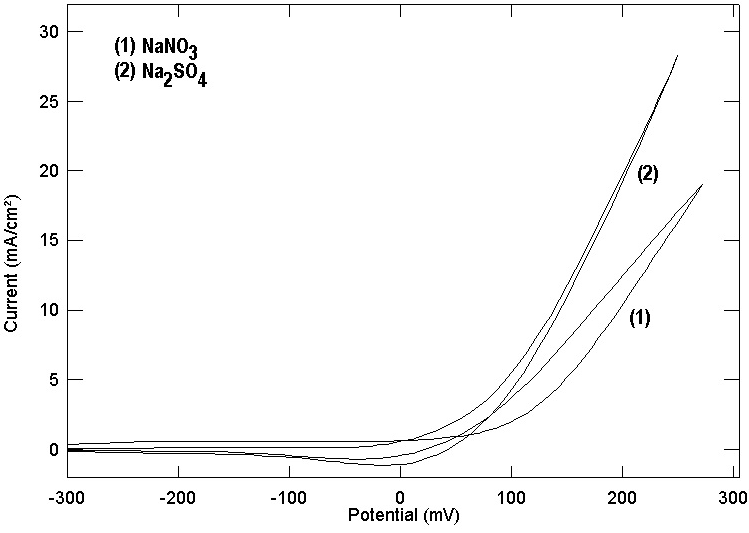 | Figure 7. Cyclic voltamogram for copper in 0.5 M aqueous solution of NaNO3 and Na2SO4 |
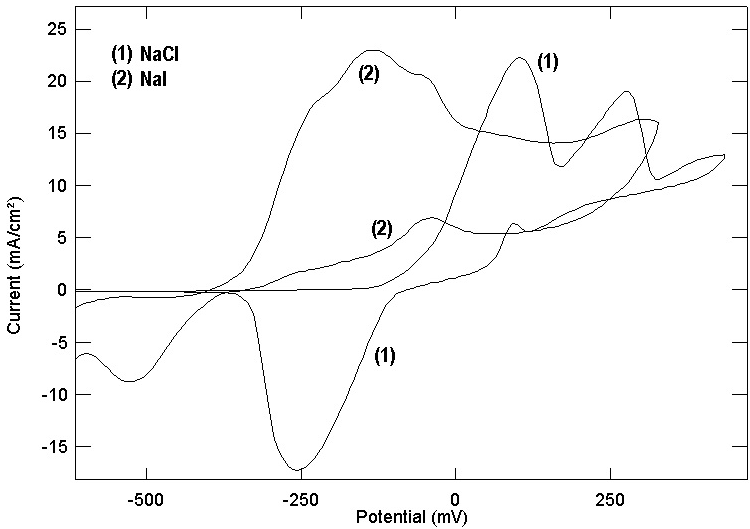 | Figure 8. Cyclic voltamogram for copper in 0.5 M aqueous solution of NaCl and NaI |
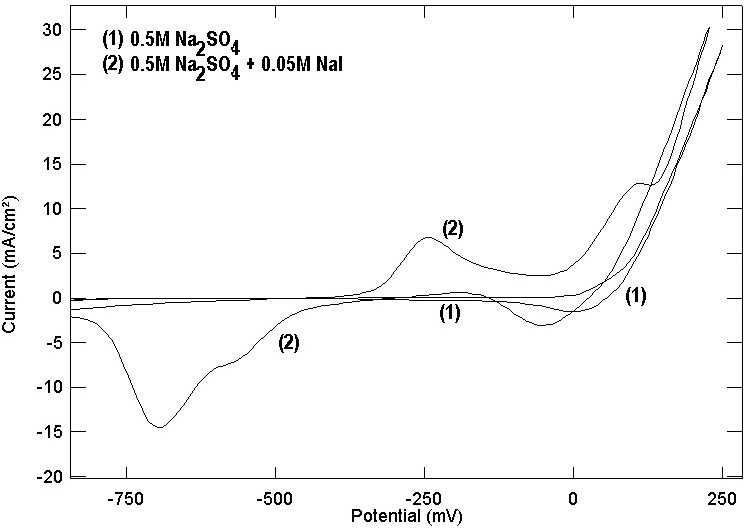 | Figure 9. Cyclic voltamogram for copper in aqueous solution of 0.5M Na2SO4 and 0.5M Na2SO4 + 0.05M NaI |
3.2. Effect of Anion on the Inhibition of the Corrosion of Copper by Diethyl Dithiocarbamate (DEDTC)
- Dithiocarbamates, the half amides of dithiocarbonic acids, are a class of monoanionic 1, 1-dithio ligands. The structure of dithiocarbamate group represented by the valence bond formalism is shown in Fig (10). The thioureide form (c) results from the delocalization of nitrogen lone pair [44].
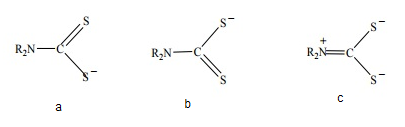 | Figure 10. Resonance forms of dithiocarbamates |
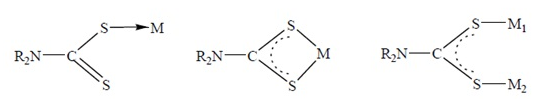 | Figure 11. Modes of bonding of the dithiocarbamates |
3.2.1. Potentiodynamic Polarization Results
- Figures (12, 13) show the polarization curves of copper in 0.5M solutions of NaNO3, Na2SO4, NaCl and NaI containing different concentrations of DETDC, the graphs show that it behaves as mixed type inhibitor. Qafsaoui et al [27] reported that pyrollidine dithiocarbamate adsorbed rabidly on copper surface, showed mixed inhibition effect and form complex through the sulfur atom. As seen in figure (12), the anodic polarization curves of copper in NaNO3 and Na2SO4 in absence of DEDTC contain one oxidation process corresponds to Cu
 Cu (II). On adding DEDTC to the electrolyte solutions, the corrosion potential of copper shifts to more negative values and oxidation of copper takes place trough two consecutive one-electron transfer steps. This behaviour can be discussed on the same basis which reported in the first part of this work. DEDTC anion contains nitrogen and sulfur atoms and also has a negative charge, therefore it is a very strong adsorbable anion and its adsorption at Cu/solution interface creates a negative charge in the diffuse double layer, this enhance the uptake of the cuprous ion from the copper surface to the solution forming [Cu (I)-DEDTC] complex. Formation of the dithiocarbamate complex with cuprous ion has been confirmed previously using the XPS technique [29]. Figure (12) shows also that increasing the concentration of DEDTC in the solution leads to a shift of the potential of the first oxidation step to more negative values, however the potential of the second oxidation step [Cu (I)-DEDTC]
Cu (II). On adding DEDTC to the electrolyte solutions, the corrosion potential of copper shifts to more negative values and oxidation of copper takes place trough two consecutive one-electron transfer steps. This behaviour can be discussed on the same basis which reported in the first part of this work. DEDTC anion contains nitrogen and sulfur atoms and also has a negative charge, therefore it is a very strong adsorbable anion and its adsorption at Cu/solution interface creates a negative charge in the diffuse double layer, this enhance the uptake of the cuprous ion from the copper surface to the solution forming [Cu (I)-DEDTC] complex. Formation of the dithiocarbamate complex with cuprous ion has been confirmed previously using the XPS technique [29]. Figure (12) shows also that increasing the concentration of DEDTC in the solution leads to a shift of the potential of the first oxidation step to more negative values, however the potential of the second oxidation step [Cu (I)-DEDTC]  [Cu (II)-(DEDTC)2] is constant and independent on the amount of DEDTC. This phenomenon can be interpreted on the basis that, increasing the concentration of DEDCT leads to increasing the value of the negative charge on the diffuse double layer at the metal/solution interface causing more enhancement of the uptake of the cuprous ion from the metal surface to the solution side. Kendig et al [49] attributed the corrosion inhibition of Cu-containing alloy AA2024 by diethyl dithiocarbamate to the formation of an insoluble Cu (II)-chelate film.
[Cu (II)-(DEDTC)2] is constant and independent on the amount of DEDTC. This phenomenon can be interpreted on the basis that, increasing the concentration of DEDCT leads to increasing the value of the negative charge on the diffuse double layer at the metal/solution interface causing more enhancement of the uptake of the cuprous ion from the metal surface to the solution side. Kendig et al [49] attributed the corrosion inhibition of Cu-containing alloy AA2024 by diethyl dithiocarbamate to the formation of an insoluble Cu (II)-chelate film.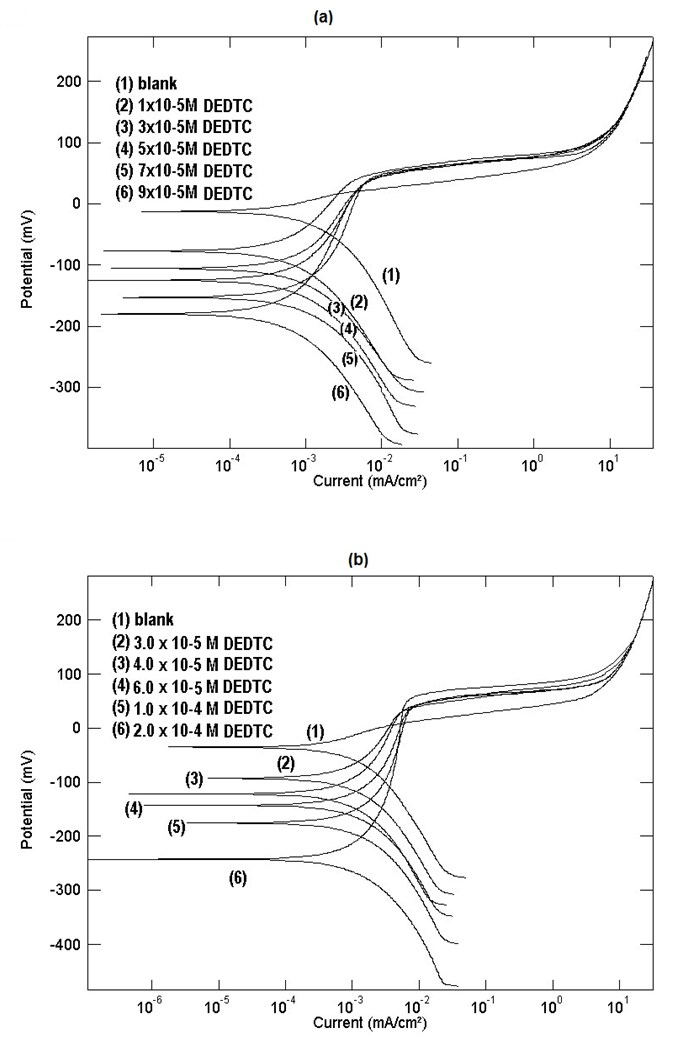 | Figure 12. Potentiodynamic polarization curves of copper in 0.5 M aqueous solution of a) NaNO3 b) Na2SO4 in absence and presence of different concentrations of DEDTC |
 Cu (I) and Cu (I)
Cu (I) and Cu (I)  Cu (II) and addition of DEDTC has no effect on the corrosion potential of copper. This behavior can be discussed on the basis that I- ion is a strong adsorbable anion and prevent the adsorption of the DEDTC anion at the Cu/solution interface.
Cu (II) and addition of DEDTC has no effect on the corrosion potential of copper. This behavior can be discussed on the basis that I- ion is a strong adsorbable anion and prevent the adsorption of the DEDTC anion at the Cu/solution interface.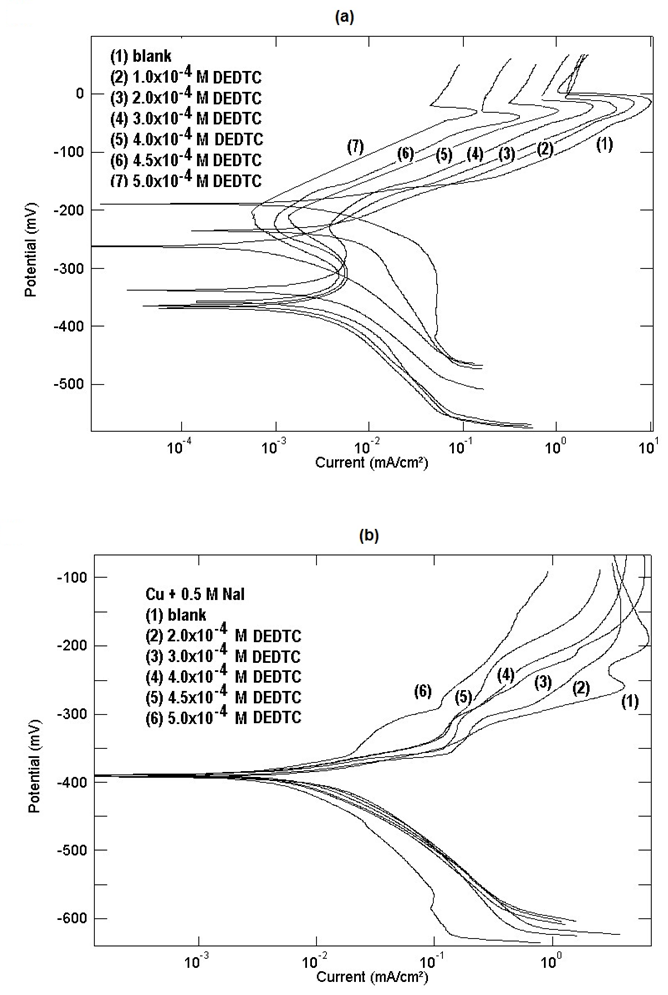 | Figure 13. Potentiodynamic polarization curves of copper in 0.5 M aqueous solution of a) NaCl b) NaI in absence and presence of different concentrations of DEDTC |
3.2.2. Electrochemical Impedance Spectroscopy (EIS) Results
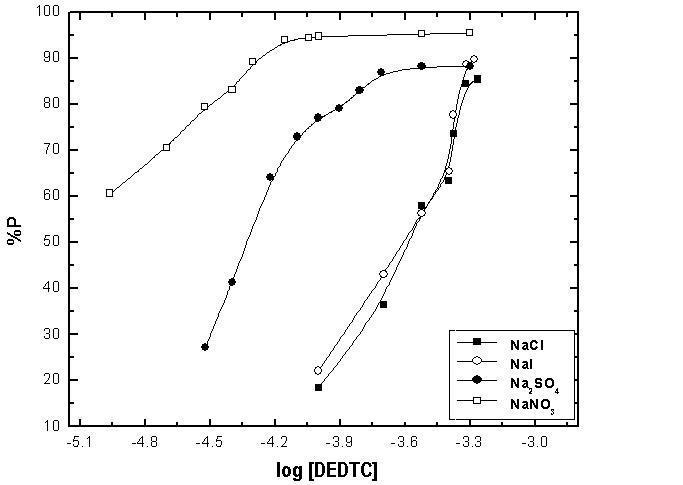
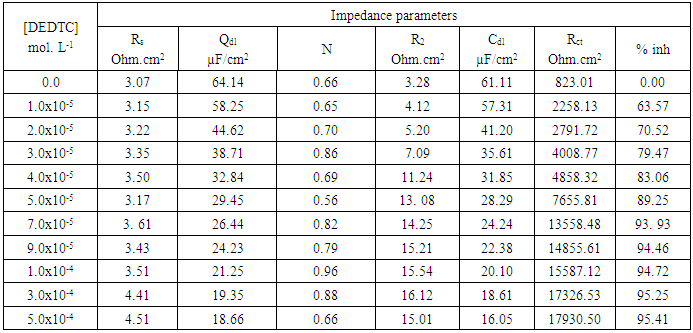
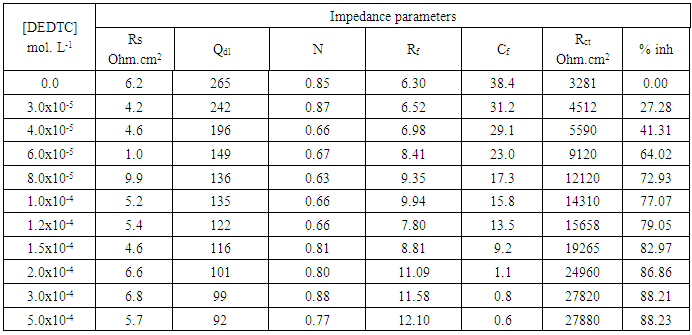
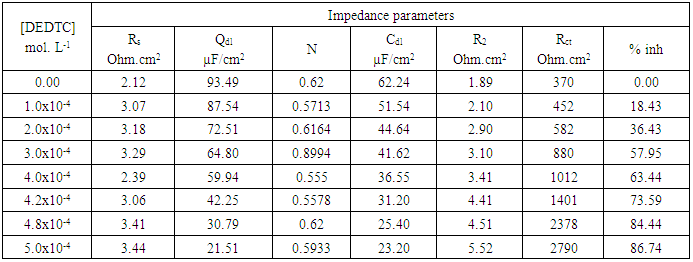
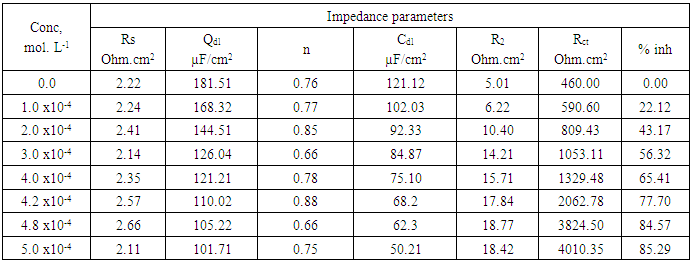
- Figures (14-17) show the Nyquist plots of copper in 0.5M solutions of each of NaNO3, Na2SO4, NaCl and NaI in absence and presence of different concentrations of sodium diethyl dithiocarbamate. Most of the Nyquist plots contain two capacitive semicircles. The capacitive semicircle at low frequency corresponds to the double layer capacity and charge transfer resistance, while the capacitive semicircle at high frequency is attributed to the corrosion products formed during the corrosion process [43]. All impedance spectra were analyzed by fitting the experimental data to the equivalent circuit model shown in figure 2. The inhibition efficiency (%P) of DEDTC was calculated from the impedance measurements using the relation [51].
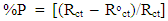 | (3) |
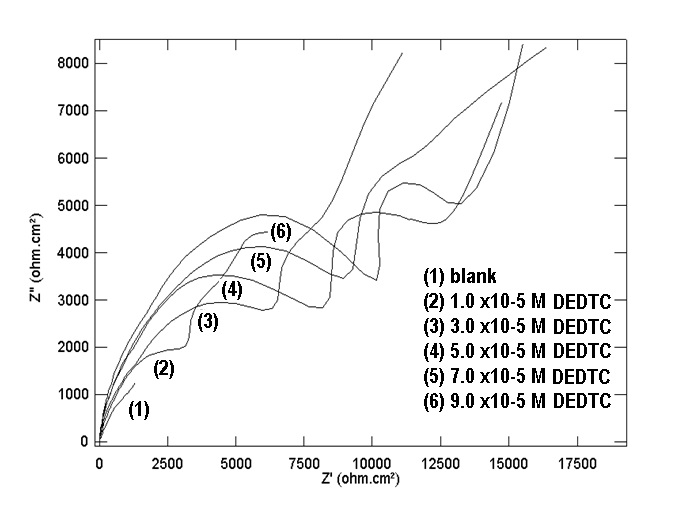 | Figure 14. Nyquist plots of copper in 0.5 M aqueous solution of NaNO3 in absence and presence of different concentrations of DEDTC |
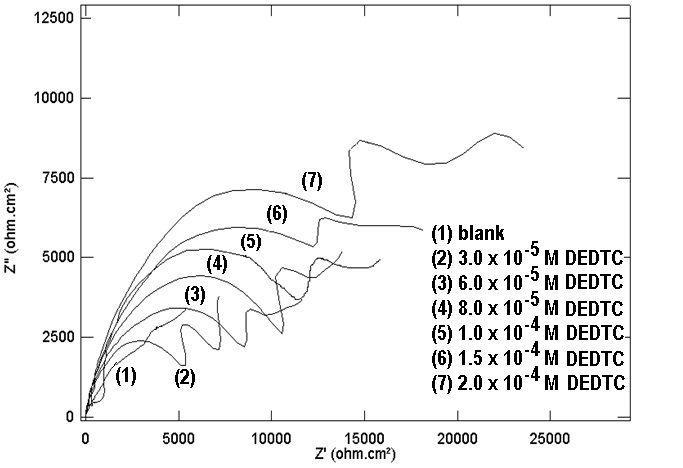 | Figure 15. Nyquist plots of copper in 0.5 M aqueous solution of Na2SO4 in absence and presence of different concentrations of DEDTC |
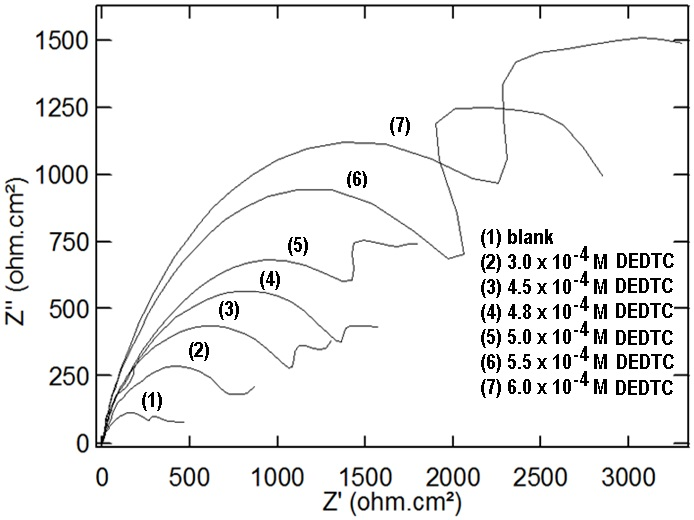 | Figure 16. Nyquist plots of copper in 0.5 M aqueous solution of NaCl in absence and presence of different concentrations of DEDTC |
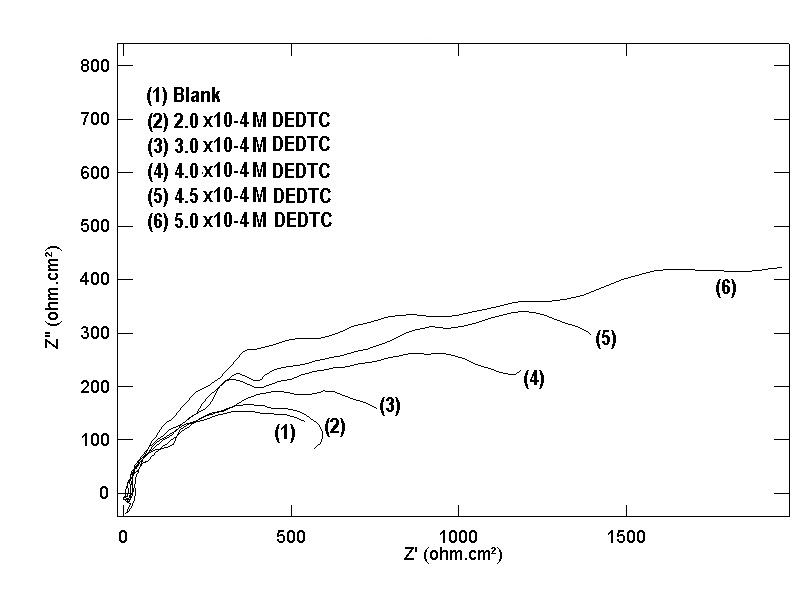 | Figure 17. Nyquist plots of copper in 0.5 M aqueous solution of NaI in absence and presence of different concentrations of DEDTC |
|
|
|
|
3.2.3. Application of Adsorption Isotherms
- Langmuir isotherm and Kinetic-thermodynamic model [52] were used to fit the corrosion data of copper in the four studied electrolytes in presence of different concentrations of DEDTC. Langmuir isotherm is given by:
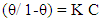 | (4) |
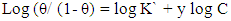 | (5) |
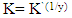 | (6) |
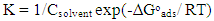 | (7) |
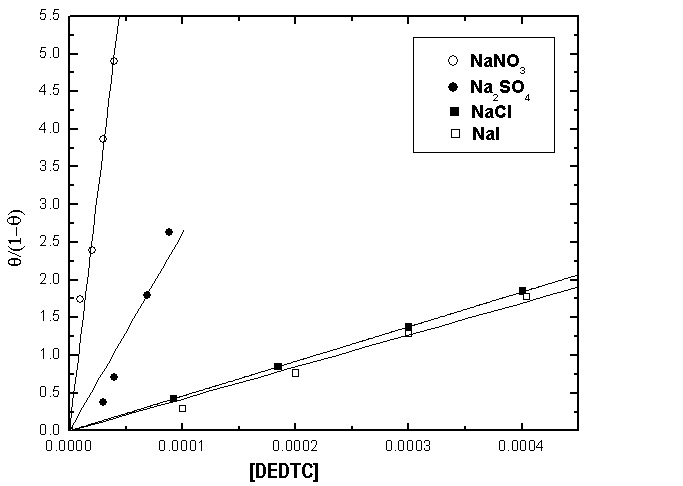 | Figure 19. Langmuir adsorption isotherm of copper in 0.5 M aqueous solution of NaNO3, Na2SO4, NaCl and NaI in presence of different concentrations of DEDTC |
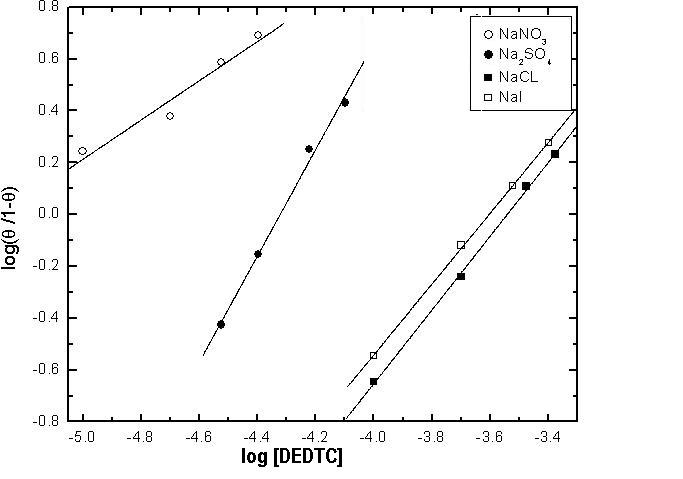 | Figure 20. Kinetic-thermodynamic model for copper in 0.5 M aqueous solution of NaNO3, Na2SO4, NaCl and NaI in presence of different concentrations of DEDTC |
|
4. Conclusions
- The results of this study show that:1- The electrochemical dissolution of copper in aqueous solutions greatly depends on the kind of anion. Oxidation of copper takes place through a two-electron transfer step Cu
 Cu(II) in electrolytes contain non- or weak adsorbable anions, however oxidation goes through two consecutive one electron transfer steps Cu
Cu(II) in electrolytes contain non- or weak adsorbable anions, however oxidation goes through two consecutive one electron transfer steps Cu  Cu(I) and Cu(I)
Cu(I) and Cu(I)  Cu(II) in electrolytes contain anions strongly adsorbed at metal/solution interface.2- Diethyl dithiocarbamate act as mixed type inhibitor in the four studied electrolytes and its inhibition efficiency depends on the kind of the electrolyte, its inhibition efficiency in electrolytes contain strong adsorbable anions is lower than that contain weak or non-adsorbable anions due to the competitive adsorption between the anions of the electrolytes and that of DEDTC at metal-solution interface.3- Both Langmuir isotherm and Kinetic-thermodynamic model are applicable to fit the experimental data of adsorption of DEDTC on the copper surface in the four examined electrolytes. The results showed that the value of the standard free energy change of adsorption (∆Goads) equal (-35.55 to -39.73 kj/mol.) indicating that the adsorption process of DEDTC on the copper surface is comprehensive (chemical and physical adsorption).
Cu(II) in electrolytes contain anions strongly adsorbed at metal/solution interface.2- Diethyl dithiocarbamate act as mixed type inhibitor in the four studied electrolytes and its inhibition efficiency depends on the kind of the electrolyte, its inhibition efficiency in electrolytes contain strong adsorbable anions is lower than that contain weak or non-adsorbable anions due to the competitive adsorption between the anions of the electrolytes and that of DEDTC at metal-solution interface.3- Both Langmuir isotherm and Kinetic-thermodynamic model are applicable to fit the experimental data of adsorption of DEDTC on the copper surface in the four examined electrolytes. The results showed that the value of the standard free energy change of adsorption (∆Goads) equal (-35.55 to -39.73 kj/mol.) indicating that the adsorption process of DEDTC on the copper surface is comprehensive (chemical and physical adsorption). Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML