-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Physical Chemistry
p-ISSN: 2167-7042 e-ISSN: 2167-7069
2017; 7(2): 27-30
doi:10.5923/j.pc.20170702.01

Environmental Remediation Using Polystyrene/ 4-Aminophenyl Methyl Sulfone and Carbon Nanotube Nanocomposite
Ayesha Kausar
Nanoscience and Technology Department, National Centre for Physics, Islamabad, Pakistan
Correspondence to: Ayesha Kausar, Nanoscience and Technology Department, National Centre for Physics, Islamabad, Pakistan.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this research effort, polystyrene (PS) nanocomposite films with 4-aminophenyl methyl sulfone (AMS) and AMS/carbon nanotube (CNT) have been fabricated for environmental remediation studies. Biodegradation of plastics as well as toxic ion removal from industrial waste water are important environmental issues. Soil burial studies of PS/AMS films have shown 40% degradation after 60 days; however nanotube addition prevented the degradation. The percent metal ion (As5+) sorption was found to increase as a function of time and nanotube content. On the other hand, PS/AMS possess lower toxic ion uptake depicting the effect of carbon nanotube. Swelling studies were also carried out on PS film with AMS additive and composite films with AMS/CNT in aqueous medium. Carbon nanotube also prevented the swelling behavior of these films. The novel nanocomposites were potentially important to resolve the plastic biodegradation and environmental pollution issues.
Keywords: Polystyrene, CNT, Arsenic, Sorption, Biodegradation
Cite this paper: Ayesha Kausar, Environmental Remediation Using Polystyrene/ 4-Aminophenyl Methyl Sulfone and Carbon Nanotube Nanocomposite, Physical Chemistry, Vol. 7 No. 2, 2017, pp. 27-30. doi: 10.5923/j.pc.20170702.01.
Article Outline
1. Introduction
- Polystyrene (PS) is an exclusive thermoplastic polymer having wide range of industrial applications [1-5]. PS shows several beneficial distinctiveness’s such as chemical inertness, low cost, processability, and ease of fabrication [6-9]. Carbon nanotube is widely used nanofiller in polystyrene nanocomposites. Iijima (1991) discovered concentric layered microtubule made up of carbon atoms [10]. These layered microtubules are filamentous carbon known as carbon nanotube (CNT). These are one of the most widely investigated carbon nanostructures [11, 12]. CNT possess large specific surface area and unique electronic structure and hybridization. Gas separation, catalyst supports, electrodes, and water purification are important areas of CNT’s applications [13-15]. CNT exists as single and multi-walled carbon nanotube. Single-walled carbon nanotube (SWCNT) is a sheet of atomically thin carbon rolled to form a cylinder. The diameter of SWCNT is 1 nm while the length is million time longer. Double-walled carbon nanotube (DWCNT) is also a type of nanotube. In multi-walled carbon nanotube (MWCNT), several graphene layers are rolled in concentric cylinders having interlayer spacing of 0.34 nm. Diameter of is few nm while length ranges up to tens of microns. Mechanical strength, electrical conductivity, electromagnetic interference shielding are important properties of MWCNT [16, 17]. Incorporation of polar functionalities in PS induces hydrophilicity to the backbone [18]. In other words, functional additives may cause induction of biodegradation of plastics. Another important use of polystyrene and carbon nanotube is in metal ions sorption for water pollution remediation. In this regard, different polymer resin and nanofiller-based membranes have been reported [19-21]. Keeping in view the environmental pollution caused by the waste polystyrene, a major objective of this work was to study its biodegradation. Second objective was to make polystyrene environmentally important. Therefore, polystyrene films with 4-aminophenyl methyl sulfone and CNT have been prepared. Thereafter biodegradation studies by soil burial method have been performed. Moreover, swelling and metal ion sorption (arsenic uptake) of nanocomposite was studied for water pollution alleviation technology.
2. Experimental
2.1. Materials
- Polystyrene (Mw ~350,000), carbon nanotube, multi-walled (>98 % carbon basis), 4-aminophenyl methyl sulfone (AMS, 97%), and tetrahydrofuran (THF, anhydrous, 99.9%) were obtained from Aldrich.
2.2. Measurement
- Swelling studies were performed to see the effect of filler on the absorption properties of films. Metal ion sorption studies were performed to study the percent metal ion uptake (arsenic ions). Soil burial studies were performed to study film biodegradation.
2.3. Annealing of MWCNT
- The pristine multi-walled carbon nanotube was weighed and annealed at 500°C for 0.5 h to remove the amorphous carbon content before use [5, 6].
2.4. Formation of Polystyrene Film
- For the preparation of neat PS film, initially 0.8 g PS was dissolved in 8 mL THF and stirred for 6 h. The polymer solution was added drop wise on a glass slide to form a layer. The film was prepared by drying at 60°C [2].
2.5. Formation of Polystyrene Film with 4-aminophenyl Methyl Sulfone and CNT
- For the preparation of nanocomposite films, 0.8 g PS was dissolved in 8 mL THF and stirred for 6 h. Afterwards, different weight ratios of CNT and AMS were added and films were caste (Table 1). The film was prepared by drying at 60°C [15, 16].
|
3. Results and Discussion
3.1. Swelling Studies
- Polystyrene film with aminophenyl methyl sulfone additive and three different composite films with CNT have been prepared. Swelling studies of the PS film with AMS additive and composite films with AMS and CNT were carried out in aqueous medium. Known weight of films were taken and immersed in excess of solvent for 24 h at fixed temperature (30°C) to attain equilibrium swelling. Then films were taken out and wiped with tissue paper to remove excess of solvent, and weighed. Percent swelling (%S) of the films was calculated as:Percent swelling (%S) = (W2-W1)/W1×100where W2 = weight after soaking and W1 = weight before soaking or dry weight. It was observed from Fig. 1 that in the case of PS/AMS swelling was higher as compared to the composite films. Minimum swelling was observed in the case of the 5 wt.% CNT loading. The results showed that the crosslinking density was increased while film porosity was decreased with the filler loading [19].
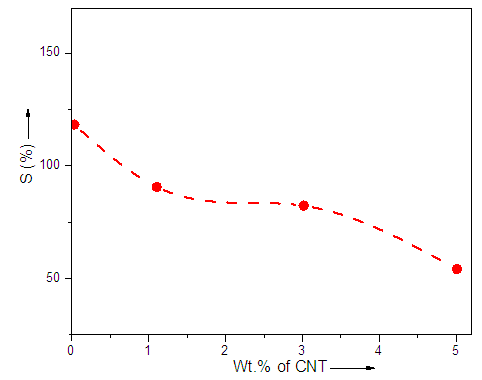 | Figure 1. Effect of filler loading (AMS and AMS/CNT) on polystyrene (Sample explanation given in Table 1) |
3.2. Metal Ion Sorption
- The percent metal ion (As5+) uptake as function of time with filler content is shown in Fig. 2. The coal mine wastewater was tested for the removal of ions. The metal ion sorption capacity of PS/AMS and composites was found to increase with the increase in sorption time. Maximum absorption in all the samples was observed after 100 min. However, there was a little decrease in the sorption time for PS/AMS/CNT 5 sample. This is because of the restricted diffusion of the ions through the polymer/CNT network and reduced chain flexibility. A series of batch adsorption studies of As5+ using nanocomposite was carried out in 100 mL Erlenmeyer flask containing 20 mL of working solution. Optimization of As5+ removal was designed with different process parameters. The equation used is given as:Removal (%) = C1-C2/C1×100Where C1 and C2 are initial and final metal concentrations (mg/L). PS/AMS/CNT 5 with higher nanotube content showed 99% As5+ removal.
3.3. Biodegradation Studies
- From the 2 months soil burial studies, it has been observed that some degradation has been occurred in the PS/AMS (Fig. 3). It was observed that 40% degradation occurred after 60 days (Fig. 3A). This is because neat polymer was more susceptible to bacterial and fungal degradations in soil for their growth. However, no degradation has been observed in the case of PS/AMS/CNT 5 with higher nanotube content. About 10% degradation was however observed in the case of 1 wt.% CNT loaded sample (Fig. 3B). That means that higher nanofiller was more effective to avoid biodegradation of polystyrene (Fig. 3C). The effect of AMS and CNT can also be studied on the biodegradation of several other polymer and composite systems [22-27].
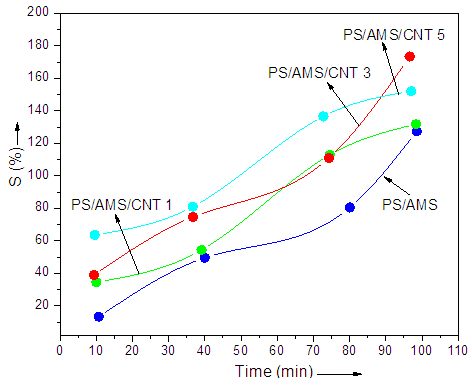 | Figure 2. Effect of sorption time on percent arsenic uptake |
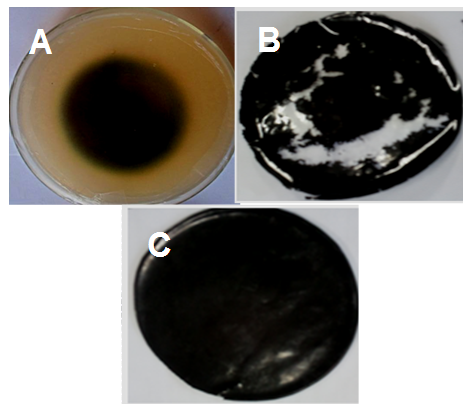 | Figure 3. Micrographs of (A) PS/AMS without CNT; (B) PS/AMS/CNT 1; and (C) PS/AMS/CNT 5 after degradation assays |
4. Conclusions
- From the results it has been concluded that the polystyrene modified with AMS showed hydrophilicity and degradability characteristics. However, these characteristics were decreased by the addition of carbon nanotube. From metal ion sorption study it is obvious that CNT formed some network with polystyrene, which can be used used for the removal and separation of hazardous metal ions from aqueous solutions. Therefore, the novel polystyrene composite with 4-aminophenyl methyl sulfone and CNT can play an important role for environmental remediation of municipal and industrial wastewaters.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML