-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Physical Chemistry
p-ISSN: 2167-7042 e-ISSN: 2167-7069
2017; 7(1): 17-26
doi:10.5923/j.pc.20170701.03

Effect of Tween-80 Surfactant on the Corrosion Resistance of Zn-Phosphated Steel
B. A. Abd-El-Nabey1, S. El-Housseiny1, H. M. El-Kshlan2, M. A. Abd-El-Fatah2
1Faculty of Science, Chemistry Department, Alexandria University, Alexandria, Egypt
2Faculty of Education, Alexandria University, Alexandria, Egypt
Correspondence to: B. A. Abd-El-Nabey, Faculty of Science, Chemistry Department, Alexandria University, Alexandria, Egypt.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

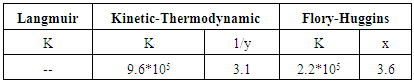
Potentiodynamic polarization, electrochemical impedance spectroscopy, SEM study and EDX analysis have been used to investigate effect of Tween-80 surfactant on the corrosion resistance, porosity, structure and composition of Zn-phosphate coat on steel. Electrochemical results indicated that the presence of 0.01M Tween-80 in the coating solution caused a decrease in the porosity and an increase of the protection efficiency of the coat up to 90%. The results proved that the coating process was controlled by adsorption of the surfactant molecules on the steel surface. Application of the adsorption isotherms and Kinetic-Thermodynamic model to fit the experimental data indicated that Langmuir isotherm is not applicable, however, Flory-Huggins isotherm and Kinetic-Thermodynamic model are applicable, and Tween-80 is chemisorbed on the steel surface. SEM study and EDX analysis gave very good support to the electrochemical data.
Keywords: Steel, Conversion Coating, Zn-Phosphate, Surfactant, EIS, Polarization, SEM, EDS
Cite this paper: B. A. Abd-El-Nabey, S. El-Housseiny, H. M. El-Kshlan, M. A. Abd-El-Fatah, Effect of Tween-80 Surfactant on the Corrosion Resistance of Zn-Phosphated Steel, Physical Chemistry, Vol. 7 No. 1, 2017, pp. 17-26. doi: 10.5923/j.pc.20170701.03.
Article Outline
1. Introduction
- Due to its excellent strength, low-cost, ability to be recycled, and good mechanical workability, steel is one of alloys are inevitable in our day to day life. Steels have a major disadvantage in that they tend to corrode [1].Corrosion can be prevented using several ways. Control of corrosion through the use of conversion coatings, is the most effective method and used in a wider range of applications [2-6]. Conversion coatings can be attained by electrochemical or chemical reaction at the steel/solution interface to form a protective film, which is more resistant to corrosion than the original metal [6]. Phosphating is one of the most widely coating processes for ferrous and nonferrous metals. Due to its low coast, easy mass production, and afford adhesion, lubricative properties, wear resistance and excellent corrosion resistance, it is widely used in the automobile, process and appliance industries [7]. Due to the high toxicity of chromate conversion coatings, Phosphate conversion coatings have been good alternative. There are several types of phosphate conversion coatings on steel [8]. Surfactants are found in a multitude of domains, and a part of daily lives [9]. Due to the ability of the surfactants to adsorb on the surfaces, they should be effective corrosion inhibitors. The surfactant inhibitors have many advantages such as easy production, lower toxicity, lower price and high inhibition efficiency [10]. The inhibition characteristics of three novel synthesized amido-amine double tailed cationic surfactants [11] and cetyltrimethyl ammonium bromide surfactant [12] have been investigated in our laboratory. Mass loss, potentiodynamic polarization and electrochemical impedance spectroscopy techniques were used to determine the efficiency of these surfactants in the inhibition of the acidic corrosion of steel. Thermodynamics of the adsorption of these surfactants and their effects on the kinetics of the dissolution reaction of steel in acid solutions have been discussed. Surfactants have a great influence on the growth of crystals and have the ability to alter crystallization kinetic [13]. This property can be effectively used to produce coatings with desirable properties. Recently, we studied the effect of Tween-80 surfactant on the electropolymerization and corrosion performance of polyaniline on carbon steel [14]. It has been found that the presence of Tween-80 in the polymerization medium inhibited the oxidation of each of steel and aniline, and the inhibition of the oxidation of steel was controlled by adsorption of the surfactant molecules at the steel/solution interface. Results obtained from the electrochemical techniques indicated that the protection efficiency of the polyaniline coat to the corrosion of steel in 0.5M H2SO4 increase to about 80% in presence of 0.025M Tween-80.In this work, we studied the effect of Tween-80 on the process of Zinc-Phosphate Coating of steel. Porosity of the coat layer and its corrosion resistance in 0.6M NH4NO3 solution were determined using potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) techniques. SEM studies and EDX analysis were also used to investigate the porosity, structure and composition of the coat. Thermodynamics of the adsorption of Tween-80 on the steel surface and its role in coating process has been also discussed.
2. Experimental
2.1. Materials and Solutions
- The stock solutions were prepared from analytical grade reagents and distilled water: 85% H3PO4, ZnO, NaNO2, and NH4NO3 was purchased from Aldrich chemicals. Tween-80 (Figure 1) was obtained from Alpha Chemika with density 1.08 g/ml. To prepare the conversion coating solution, 0.75g of ZnO, 1g of NaNO2 and 2 ml H3PO4 dissolved in certain volume of double distilled water and Tween-80 solution to give the required concentration of the surfactant.
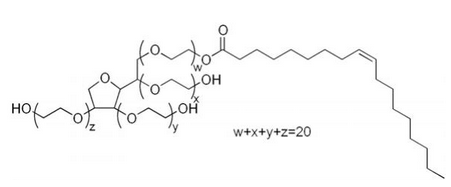 | Figure 1. Chemical structure of Tween-80 surfactant |
2.2. Application of Conversion Coating
- The steel sample was wet hand-polished using different grade emery papers 100, 320, 400, 800 and 1000 grit finishes starting with a coarse one and proceeding in steps to the fine grit up to a mirror finish, washed thoroughly with double-distilled water, then pickled, the electrode was immersed for 30 sec in ethanol for degreasing then 30 sec in 2M NaOH solution (Soda pickling) and subsequently rinsed with distilled water for 15 sec. Just before application of conversion coating, electrode was pickled with (85%) phosphoric acid at (temperature 40-50°C) and rinsed with distilled water. Then, the electrode was suspended by rotator (60 rotates per min.) in beaker containing 100 ml of the conversion coating solution for 30 min., and finally dried by air. Each experiment was carried out with newly polished, pickled electrode, and conversion coating solution.
2.3. Electrochemical Measurements
- Electrochemical measurements were recorded using frequency response analyzer (FRA)/potentiostat supplied from Parstat Instrument (PARSTAT 2263.02 SN 194). The frequency range for EIS measurements was 1.0 × 104 to 0.1 Hz with applied potential signal amplitude of 10 mV around the rest potential. The electrochemical corrosion studies in 0.6M NH4NO3 solution were carried out using a three- electrode mode cell and bare or chemically treated steel has the chemical composition as given in our previous work [12].The working electrode was introduced into the test solution before polarization and EIS measurements and left for 10 min to attain the rest potential at which the change of potential with time is 2 mV/min, i.e., the system had been stabilized. The polarization curve measurements were obtained at scan rate of 20 mV/min starting from cathodic potential (Ecorr -300 mV) going to anodic direction. All the measurements were done at 30.0 ± 0.1°C under static conditions in solutions open to the atmosphere. In order to test the measurements reproducibility and reliability, duplicate experiments were performed in each case of the same conditions.
2.4. Scanning Electron Microscope (SEM) and Energy Dispersive X-ray Spectrometer (EDS)
- A JOEL scanning electron microscope instrument was employed to characterize the surface morphology of the free and coated steel samples. The elemental composition of Zn-phosphate coat was analyzed by energy dispersive X-ray spectrometer (EDS, JEM-2100, Japan).
3. Results and Discussion
3.1. Potentiodynamic Polarization Results
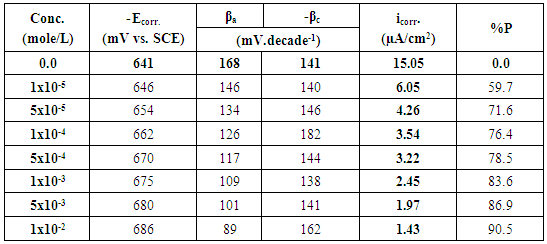
- Polarization curves of Zn-Phosphated Steel in 0.6 M NH4NO3 in solution containing different amounts of Tween-80 surfactant are presented in Figure 2. As seen from the figure, addition of the surfactant leads to the polarization of both the cathodic and the anodic curves.Table 1 shows the electrochemical polarization parameters, corrosion potential (Ecorr), anodic and cathodic Tafel line slopes (βa, and βc) and corrosion current density (icorr) for Zn-Phosphated Steel in 0.6 M NH4NO3 in absence and presence of different concentrations of Tween-80. The corrosion potential Ecorr shifts to more cathodic values with increasing the concentration of the surfactant. The value of βc is nearly constant and independent on the addition of the surfactant, while the value of βa decreases remarkably with increasing the surfactant concentration. The data revealed also that, the corrosion current density that is directly proportional to corrosion rate decreases with increasing the Tween-80 concentration. The table shows also the protection efficiency of the coat which can be calculated using the equation (1).
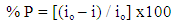 | (1) |
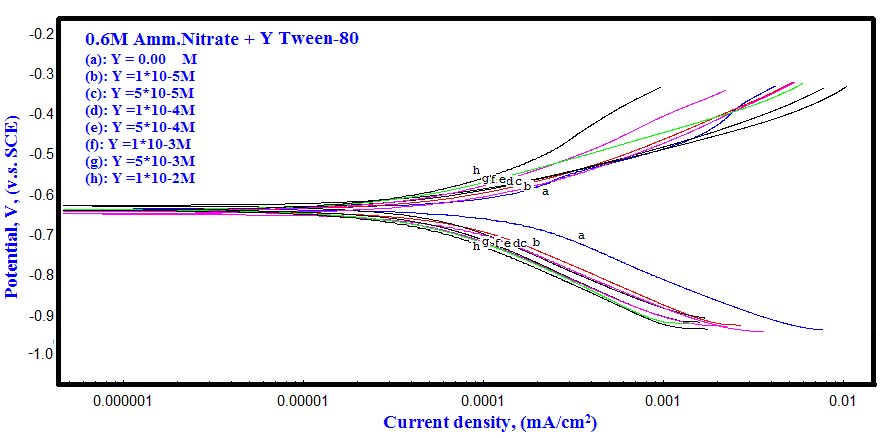 | Figure 2. Polarization curves of Zn-Phosphated Steel in 0.6 M NH4NO3 solution in absence and presence of different concentrations of Tween-80 |
3.2. Electrochemical Impedance Spectroscopy (EIS) Results
- Nyquist impedance plots of bare steel and Zn-Phosphated Steel in 0.6 M NH4NO3, in solution containing different amounts of Tween-80 are presented in Figure 3. The plots consist of distorted semicircles followed by diffusion tail indicative that the corrosion process takes place under diffusion control. The increase in the size of the semicircle in presence of the Tween-80 indicates that the phosphate coat gradually forms on the steel surface.
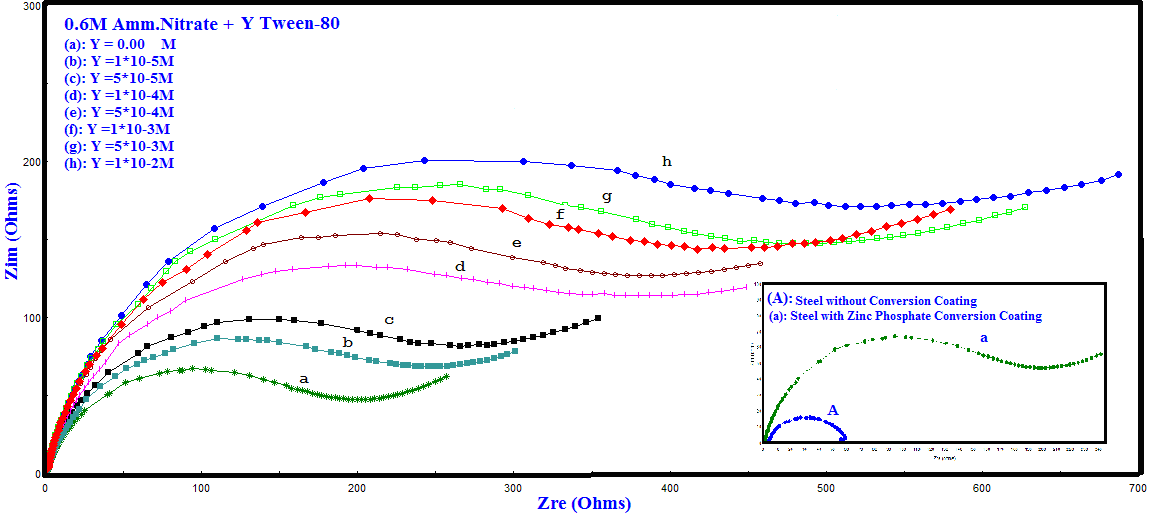 | Figure 3. Nyquist plots of bare steel and Zn-Phosphated steel in 0.6 M NH4NO3 solution in absence and presence of different concentrations of Tween-80 |
|
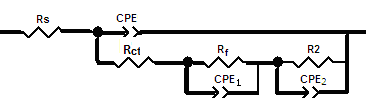 | Figure 4. Schematic for the equivalent circuit of coated steel |
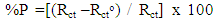 | (2) |
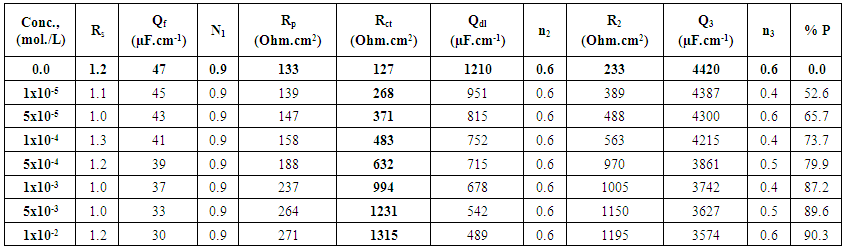 | Table 2. Electrochemical impedance parameters of Zn-Phosphated steel in 0.6 M NH4NO3 solution in absence and presence of different concentrations of Tween-80 |
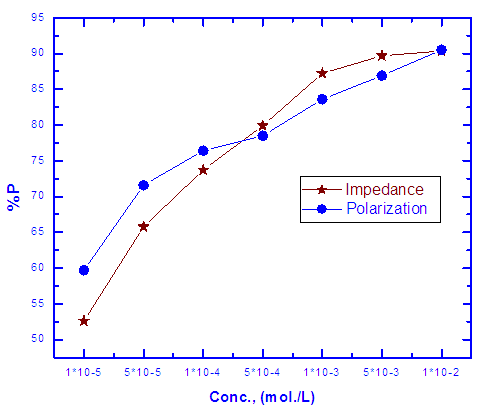 | Figure 5. Variation of the protection efficiency of Zn-Phosphate coat with concentration of Tween-80 in 0.6 M NH4NO3 solution |
3.3. Application of Adsorption Isotherms
- Adsorption of the adsorbate molecules at the metal surface was considered as simple replacement process, in which an surface active molecule from the bulk solution replace water molecule adsorbed on the metal surface, viz.
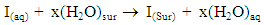 | (3) |
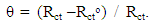 | (4) |
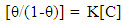 | (5) |
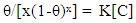 | (6) |
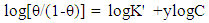 | (7) |
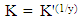 | (8) |
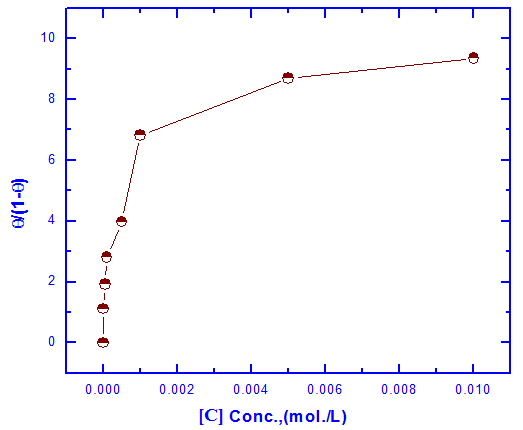 | Figure 6. Linear fitting of the data of Tween-80 to Langmuir isotherm for Zn-Phosphated Steel |
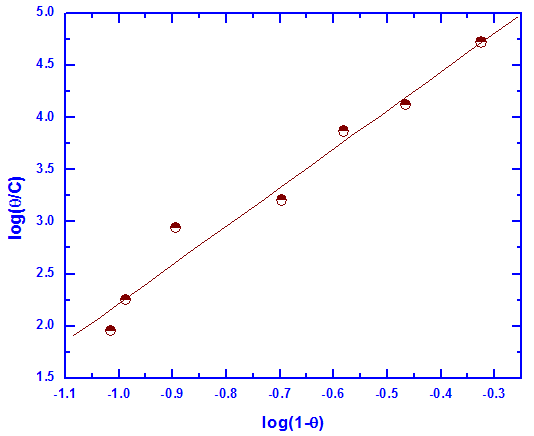 | Figure 7. Linear fitting of the data of Tween-80 to Flory Huggins isotherm for Zn-Phosphated Steel |
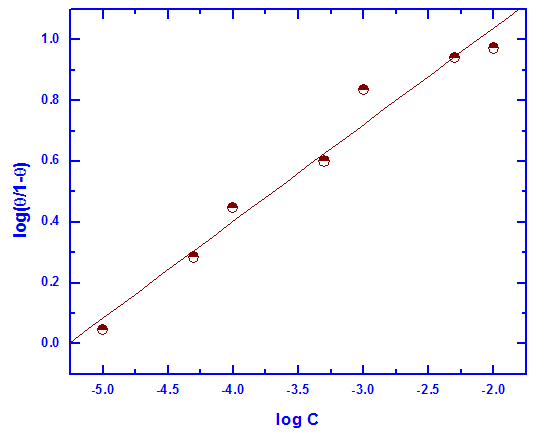 | Figure 8. Linear fitting of the data of Tween-80 to Kinetic-Thermodynamic model for Zn-Phosphated Steel |
|
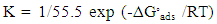 | (9) |
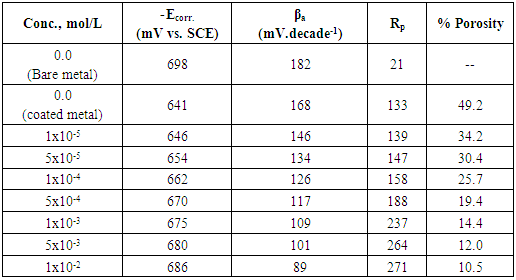
3.4. Porosity of Coat
- Phosphate conversion coating is mainly composed of insulating hopeites; the pores in the coat are generally regarded as the exposed area of the substrate. On the assumption that at low anodic overpotentials the coating is electrochemically inert, Elsener et al [25] suggested a method to estimate the porosity of thin films from the electrochemical data using equation 10.
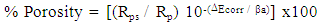 | (10) |
 0.001 mole/L. However, the presence of higher concentrations of the surfactant has a slight effect on the porosity of the coat, this behaviour can be discussed on the basis that the conversion coating process is probably controlled by the adsorption of the surfactant molecules on the steel surface. In presence of low concentrations of the surfactant the surface coverage of the steel surface with Tween-80 molecules increases sharply with the concentration of the surfactant until
0.001 mole/L. However, the presence of higher concentrations of the surfactant has a slight effect on the porosity of the coat, this behaviour can be discussed on the basis that the conversion coating process is probably controlled by the adsorption of the surfactant molecules on the steel surface. In presence of low concentrations of the surfactant the surface coverage of the steel surface with Tween-80 molecules increases sharply with the concentration of the surfactant until  0.001 mole/L, the concentration corresponds to the plateau of the Langmuir isotherm (Figure 6). At this point there is a saturation of steel surface and complete formation of the first layer of the adsorbed Tween-80 molecules.
0.001 mole/L, the concentration corresponds to the plateau of the Langmuir isotherm (Figure 6). At this point there is a saturation of steel surface and complete formation of the first layer of the adsorbed Tween-80 molecules.
|
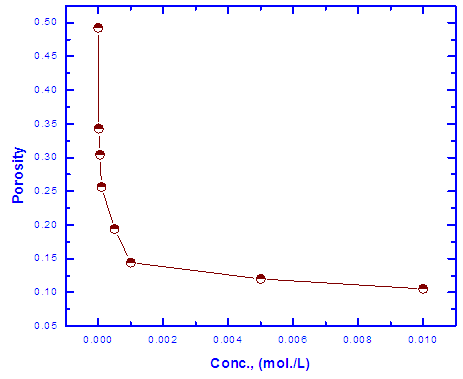 | Figure 9. Variation of the porosity of Zn-Phosphate coat with concentration of Tween-80 |
3.5. SEM Study and EDX Analysis
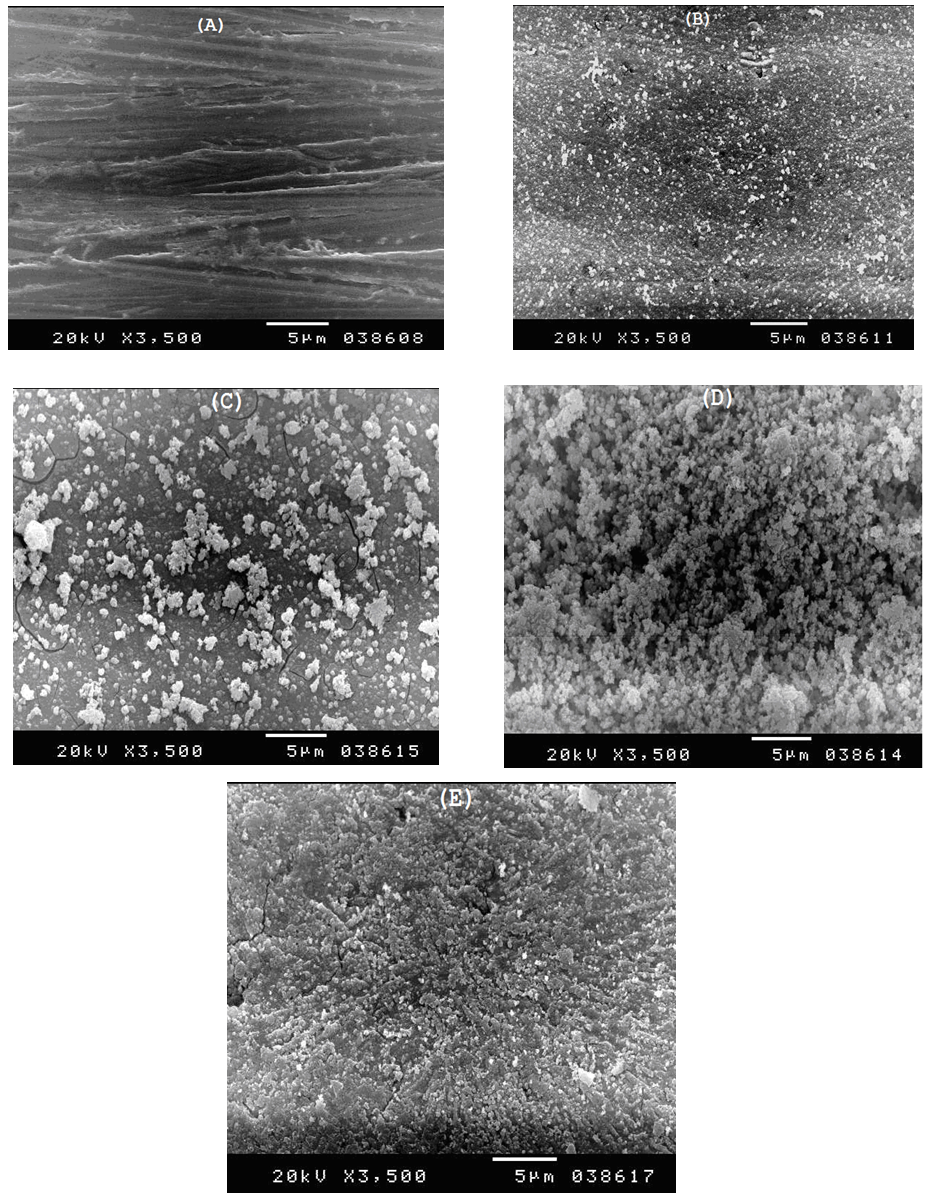
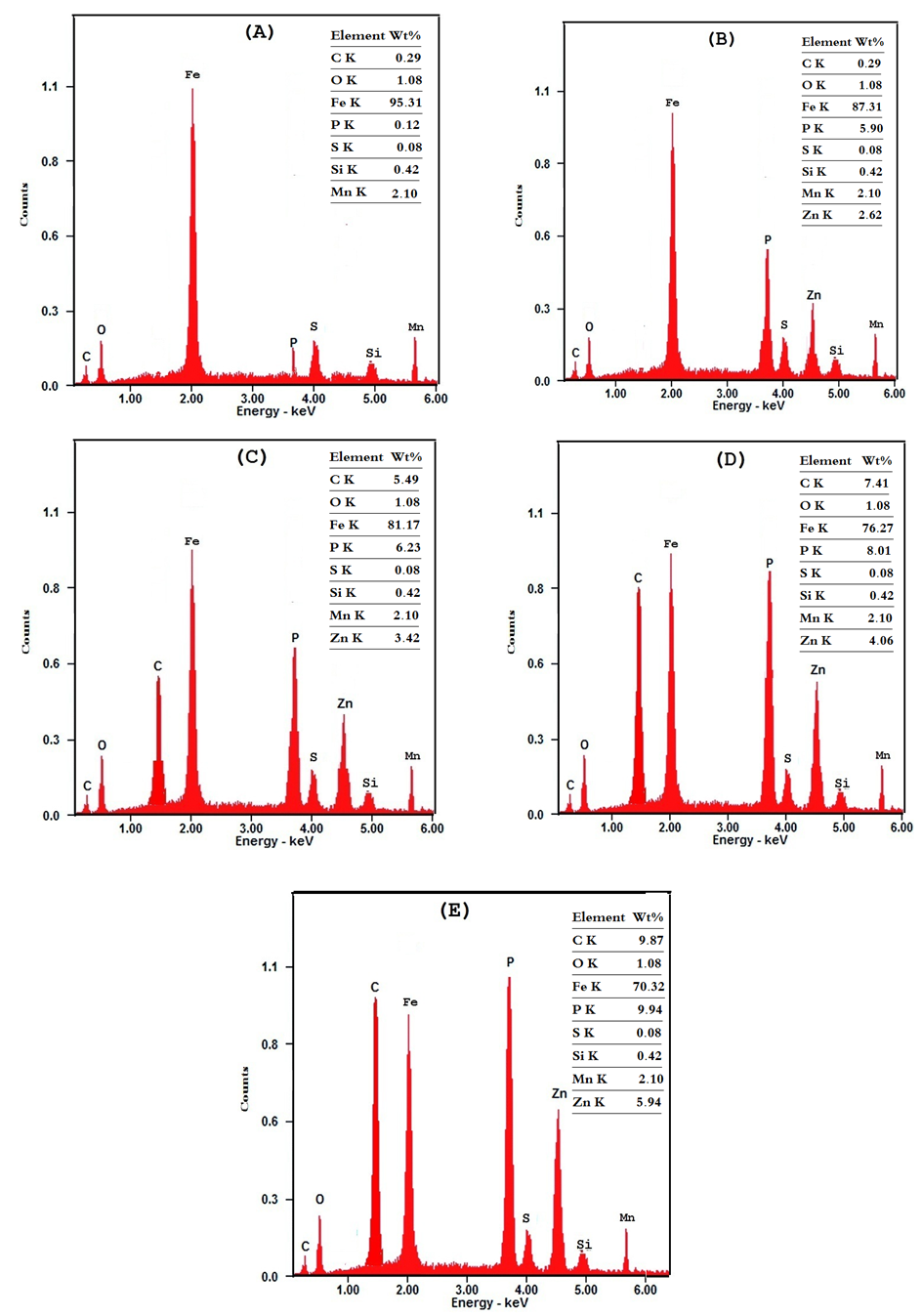
- Figure 10 shows the SEM photographs of: A) bare steel, B) Converted coated steel, C, D, E) Converted coated steel in presence of 5*10-4 M, 1*10-3M and 1*10-2 M Tween-80 respectively. It is clear that in the absence of Tween-80 during the coating process (B) the exposed area of the steel surface i.e. the porosity of the coat is large. On adding increasing amount of Tween-80 during the coating process (C, D, E), the porosity of the coat decreases and the coat layer becomes more thick and compact.The EDX analysis of the above five steel samples are given in Figure 11 and the results are summarized and presented in Figure 12. The last Figure shows the variation of wt% of each of P, Zn, and C elements with the concentration of Tween-80. The results indicating the followings: (1) Carbon is present in the coat and its percent increases with increasing the Tween-80 concentration which means that the Zn-phosphate conversion coat contains the surfactant., (2) Percent of each of Zn and P in the coat increases also with increasing the concentration of Tween-80 which indicates that the covered area of steel surface with the coat increases, while the porosity of the coat decreases with increasing the concentration of Tween-80., and (3) Dependence of the percent of the three elements on the concentration of Tween-80 shows a plateau similar to that of Langmuir isotherm indicating that adsorption of the surfactant molecules at the steel surface controls the coating process. It is clear that SEM studies and EDX analysis of Zn-phosphate coat on the steel surface gave very good support to the electrochemical data.
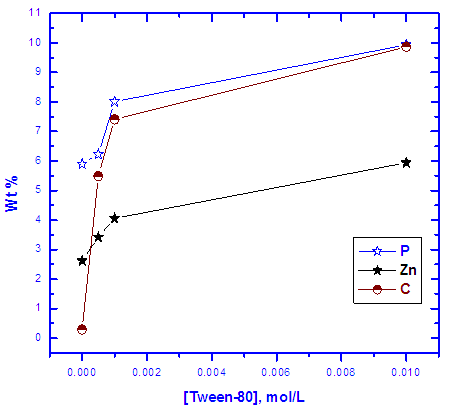 | Figure 12. Variation of the weight percent of each of C, P and Zn and the concentration of Tween-80 in coated steel surface |
4. Conclusions
- 1. Electrochemical results indicated that the presence of 0.01M Tween-80 in the coating solution caused an increase of the protection efficiency of Zn-phosphate coat on steel up to 90% and a decrease of its porosity from 49.2 to 10.5%.2. Thermodynamic study of the adsorption of Tween-80 on the steel surface indicated that Langmuir isotherm is not applicable, however, Flory-Huggins isotherm and Kinetic-Thermodynamic model are applicable, and Tween-80 is chemisorbed on the steel surface. 3. The reduction in porosity and increase in corrosion resistance of Zn-phosphate coat on steel due to the presence of Tween-80 during the coating process has been discussed on the basis of the ability of this long-chain surfactant to form films over the surface helps to seal the pores effectively, causing low moisture permeability and high corrosion-resistance properties.4. SEM studies and EDX analysis gave very good support to the electrochemical data.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML