-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Physical Chemistry
p-ISSN: 2167-7042 e-ISSN: 2167-7069
2017; 7(1): 8-16
doi:10.5923/j.pc.20170701.02

Highly Photocatalytic Activity of Novel CuFe2O4/GO Nano Composite in the Degradation of Phenol from Aqueous Solution
Giang H. Le1, Quyet T. Ngo1, Tuan T. Nguyen1, 2, Quang K. Nguyen1, Trang T. T. Quan1, Tuan A. Vu1, 2
1Institute of Chemistry, Vietnam Academy of Science and Technology (VAST), 18 Hoang Quoc Viet, Cau Giay District, Hanoi, Vietnam
2Graduate University of Science and Technology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Cau Giay District, Hanoi, Vietnam
Correspondence to: Tuan A. Vu, Institute of Chemistry, Vietnam Academy of Science and Technology (VAST), 18 Hoang Quoc Viet, Cau Giay District, Hanoi, Vietnam.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The CuFe2O4/Graphene oxide (GO) composite was successfully synthesized by co-precipitation in “situ” using FeCl3.6H2O and CuCl2.2H2O as iron and copper sources on GO and followed by thermal reduction. The product was characterized by XRD, XPS, TEM, BET and FTIR. From the XRD and XPS results, it reveals the formation of copper ferrites spinel CuFe2O4 on GO surface. From TEM images, it showed that CuFe2O4/GO composite has particle size of 20-30 nm and uniform size distribution. The as-prepared CuFe2O4/GO nano composite was used to test the photocatalytic degradation of phenol from aqueous solution. After 90 minutes of reaction, the conversion (calculated according COD) reached to the value of 90%, indicating highly photocatalytic activity of this catalyst. Moreover, after three recycles, the photocatalytic activity was almost unchanged as compared to that of fresh catalyst, indicating the relatively high stability and it can be reused. Additionally, this CuFe2O4/GO composite showed highly magnetic property so it can be easily recovered by applying the magnetic field out side.
Keywords: Nanocomposite CuFe2O4/GO, Simulated sun-light irradiation, Photocatalytic phenol degradation
Cite this paper: Giang H. Le, Quyet T. Ngo, Tuan T. Nguyen, Quang K. Nguyen, Trang T. T. Quan, Tuan A. Vu, Highly Photocatalytic Activity of Novel CuFe2O4/GO Nano Composite in the Degradation of Phenol from Aqueous Solution, Physical Chemistry, Vol. 7 No. 1, 2017, pp. 8-16. doi: 10.5923/j.pc.20170701.02.
Article Outline
1. Introduction
- In recent years, graphene based materials (graphene and graphene oxide) have received a special attention due to its unique structure and physical chemical properties as well as their utilization in a variety of applications such as electrode in batteries and super capacitors, conductive matrix, support material and adsorbents in environment [1]. Graphene, a two-dimensional nano material with several layers of sp2 hybridixed carbon atoms arranging in six-membered rings, has showed unique properties including, high Young’s modulus (ca. 1100 GPa), high strength (125 GPA), high thermal (5000 W.m-1.K-1) and electrical (0,96 x 10-6 W-1.m-1) conductivities at room temperature, high specific surface area (theoretical value 2630 m2.g-1) and high chemical stability [1, 2a-c]. Due to its can be used excellent electric conductivity and high surface area, graphene based materials as suitable material used as two-dimensional platform to accept and spread photogenerated electron –hole pairs from semiconductor upon light irradiation, enhancing the photodegradation efficiency of semiconductor in photodegradation reaction [3, 4]. The spinel structured ferrites have the general formula MFe2O4 (M: Fe, Co, Ni, Zn, Cu, etc.). The properties of the ferrite can be varied by changing the identity of the divalent M2+ cation. Among the ferrites, copper ferrite nanoparticles (CuFe2O4) has received a great interest and is widely used in electronics, and catalysis [5, 6, 7]. CuFe2O4 is one of the iron-based cubic spinel series showing advantages of a narrow band gap (1.9eV) for high absorption efficiency of sunlight, high photochemical stability, and excellent ferromagnetic properties for catalyst recovery by applying magnetic field [7]. However, CuFe2O4 nanomaterial used as a photocatalyst in the degradation of pollutants is still already investigated. However, the CuFe2O4 nanoparticles are tended to aggregate due to the ferromagnetic properties, forming large particles. Moreover, because of the narrow band-gap resulted in the rapid recombination of electrons and hole it caused the fast decreased of their photo catalytic activity.To overcome this recent years, different magnetic iron oxides graphene nano composites, have been synthesized and it claimed that the dispersion and attachment of metal oxide particles can be improved, resulting in enhance of photocatalytic performance in the degradation of organic compounds as compared with metal oxide itself or pristine graphene [3, 8, 9]. The improvement of photocatalytic performance can be explained due to the special ability of graphene, GO which can act as electron acceptor/shuttle photogenerated from metal oxide upon light irradiation. This makes life span of photoexcited electron- hole pairs longer, thereby hindering the electron-hole recombination and enhancing photoactivity of materials. Additionally, graphene oxide has abundant oxygenated functional groups such as hydroxyl, epoxides and carboxyl groups, easily formed the covalent bond with metal ions and can be more suitable to disperse CuFe2O4 nano particles on GO surface.In this paper, we report a two-step synthesis of CuFe2O4/GO composites through co-precipitation in “situ” and thermal reduction and photo fenton activity in the degradation of Phenol. Effects of pH, catalyst dosage as well as stability of the catalysts investigated.
2. Experimental
2.1. Synthesis of GO
- Graphene oxide is synthesized by chemical oxidation and exfoliation of natural graphite using modified Hummers method [10]. GO powders were exfoliated by the treatment in a microwave oven (Model MWO-G20SA) in ambient conditions at 700 W for one minute [11]. Upon microwave irradiation, the volume expansion of the GO powders much is larger than the exfoliated by using the common method (ultrasonic treatment)
2.2. Synthesis of Fe3O4/GO Nano Composite
- Magnetic iron oxide - graphene oxide composites (Fe3O4/GO) were prepared by co-precipitation using FeCl3.6H2O and FeCl2.4H2O as ion-containing sources and deposited on GO surface. Suspension of GO was diluted with distilled water under vigorous stirring. After that, the mixture of FeCl3 and FeCl2 (ratio Fe3+/Fe2+:2/1) was slowly added to the GO mixture. The mixture was adjusted solution pH value by adding 15 mL NH4OH 30% to reach the pH value of 10. The obtained gels were maintained at 80°C for 45 minute. The product was repeatedly washed by distillation water and ethanol until pH value of 7. The final product was dried in vacuum oven at 60°C before use. The ratio of magnetite to GO (in weight) is 1:3.
2.3. Synthesis of CuFe2O4/GO Nano Composite
- Nanocomposite CuFe2O4/GO was synthesized by co-precipitation in “situ” using FeCl3.6H2O and CuCl2.2H2O as iron and copper sources on GO and followed by the thermal reduction. Firstly, 225 mg GO was added into 110 mL of distilled water, ethanol solution mixture was treated in a ultrasonic bach for 30 minute. After that, 53.5 mg of CuCl2.2H2O and 169.1 mg of FeCl3.6H2O were added to 110 mL of distilled water and ethanol under stirring condition for 15 min. at room temperature. The above two mixtures were put together and stirred for 30 min. After that, the mixture was adjusted to pH value of 10 by adding 15 mL NH4OH 30% solution and stirred for 3 h. The obtained products were repeatedly washed by distillation water until pH of 7 and then freeze dried. Finally, the obtained product was calcined by heating at 650°C for 2 h in nitrogen atmosphere. The ratio of CuFe2O4/GO (in weight %) is 1:3.
2.4. Characterization
- The powder X-ray diffraction (XRD) patterns of the samples were recorded on a Shimadzu XRD-6100 analyzer with Cu Ka radiation (l¼1.5417° A). Transmission electron microscopy (TEM) using JEOL 1010 instrument operating at 80 kV with magnification of 25,000–100,000. The X-ray photoelectron spectroscopy (XPS) measurement was performed on the ESCALab MKII spectrometer using Mg Kα radiation. A vibrating-sample magnetometer (VSM, PPMS6000, Quantum Design) was used to study the magnetic properties of magnetite nano particles. The FT-IR spectra of the samples were measured by the KBr pellet method (BIO-RAD FTS-3000). Surface Area of samples was determined on Quantachrome Instruments version 3.0 at 77 K and using nitrogen adsorbed. Energy-dispersive X-ray spectroscopy (EDX) of samples were measured using JEOL JED-2300 spectrometer.
2.5. Photo-Fenton Reaction
- Pyrex glass bottles, in which a 100 mL aliquot of each phenol solution with initial concentrations of 100 mg/L was added. Photocatalytic degradation of phenol from aqueous solution was carried out in a bach reactor at the following conditions: photocatalyst conc. of 0.05 - 0.1 g/L and H2O2 conc. of 136 mg/L. Photo-Fenton reaction of phenol was performed under stirring condition, at room temperature and pH in the range of 2.5 – 8.0. The pH value of reactant solution was adjusted with HCl 0.01 M or NaOH 0.01 M solution. The samples were collected at diffirent reaction time. Simulated sunlight source was used UV-A range lamps (4 lamps, power of 15W for each lamp). The emission spectrum between 400 and 800 nm follows the solar spectrum. Before starting the illumination,each reaction mixture was stirred for 25 minute in the dark in order to reach the adsorption–desorption equilibrium between the phenol and the catalysts. Concentrations of phenol and the intermediates formed the processes were determined by using high performance liquid chromatography (HPLC – Agilent 1100) with detector DAD (Agilent - US). The assessment of the photodegradation was also examined by chemical oxygen demand (COD). The COD was measured by potassium dichromate titrimetric method by using Lambda 35 UV-vis spectrophotometer intensities of the band at 610 nm.The COD removal efficiency (H %) was calculated as following equation:
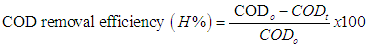 Where CODo is the COD initial of phenol and CODt is the COD after the different time.
Where CODo is the COD initial of phenol and CODt is the COD after the different time.3. Results and Discussion
3.1. X-ray Diffraction (XRD) and Fourier Transform Infrared Spectroscopy (FTIR) Analysis
- The X-ray diffraction pattern of the CuFe2O4/GO composite was shown in Fig. 1a. The diffraction peaks (30.1°, 35.4°, 43.3°, 57.2°, 62.7°) of CuFe2O4/GO corresponded to the (220), (311), (400), (511), (440) planar of face-centered cubic structure of CuFe2O4 as reported in the literature [8, 12]. The absence of the peak at 2θ of 11° (fig. 1b) which is characteristic for GO structure, indicated the intercalation of CuFe2O4 nanoparticles within GO layers. Additionally, nano CuFe2O4 particles generated on the GO surface prevent the restacking of GO sheets to form regular and periodic layered structure of graphite [13]. However, no typical peak of graphene at 2θ of 23-26,3° was observed, suggesting that the graphene sheets were exfoliated [14].The FTIR spectrum of CuFe2O4/GO was presented in Fig. 1c. The band at 3419 cm−1 is attributed to the stretching vibration of O−H. Strong bands in the region of 1620 – 1235 cm-1 correspond to the C=C stretching vibration, the C−O−H deformation vibration, respectively. The band with low intensity at 2342 cm-1 assigned to CO2 – GO bending vibrations [15]. The new bands at 530 cm−1 and 437 cm-1, appeared indicating the interaction between CuFe2O4 and the graphene oxide sheets through metal-carbonyl coordination [8].
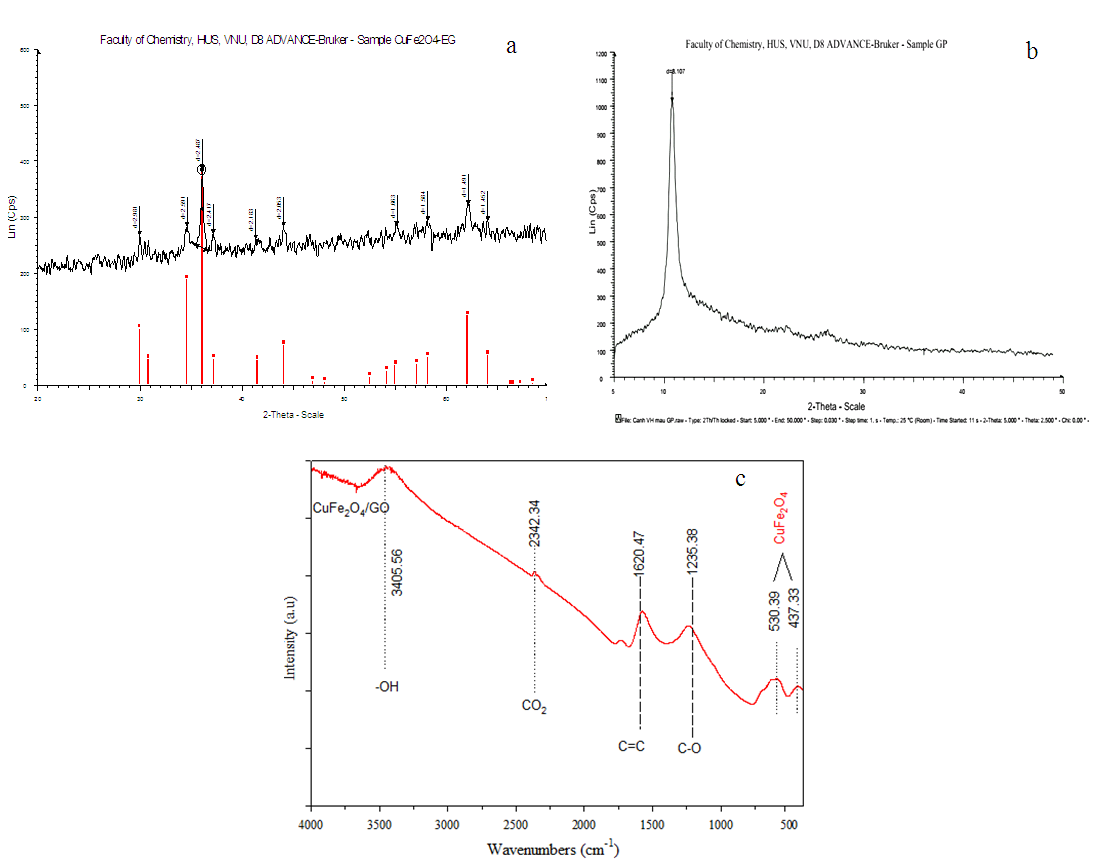 | Figure 1. XRD patterns of CuFe2O4/GO (a); GO (b) and (c) FT-IR spectra of CuFe2O4/GO |
3.2. Transmission Electron Microscopy (TEM) and Energy-dispersive X-ray Spectroscopy (EDX) Analyses
- The image of CuFe2O4 was shown in Fig. 2a. As observed in Fig. 2a CuFe2O4 nanoparticles anchored on GO sheets and relatively well distributed. It could be noted in Fig.2a that the size of the CuFe2O4 on GO was around 20–30 nm with a relatively narrow size distribution and a certain extent of particles aggregation. The elemental composition of the hybrid was examined by EDX spectroscopy and the results were present in Fig. 2b. From analysis result, it shows that CuFe2O4/GO contained 43.79% C in weight, 27.51% O in weight, 22.87% Fe in weight and 5.84% Cu in weight, while the Fe3O4/GO contained 40.89% C in weight, 34.75% O in weight and 24.20% Fe in weight, respectively. This revealed that CuFe2O4 and Fe3O4 have the similar composition.
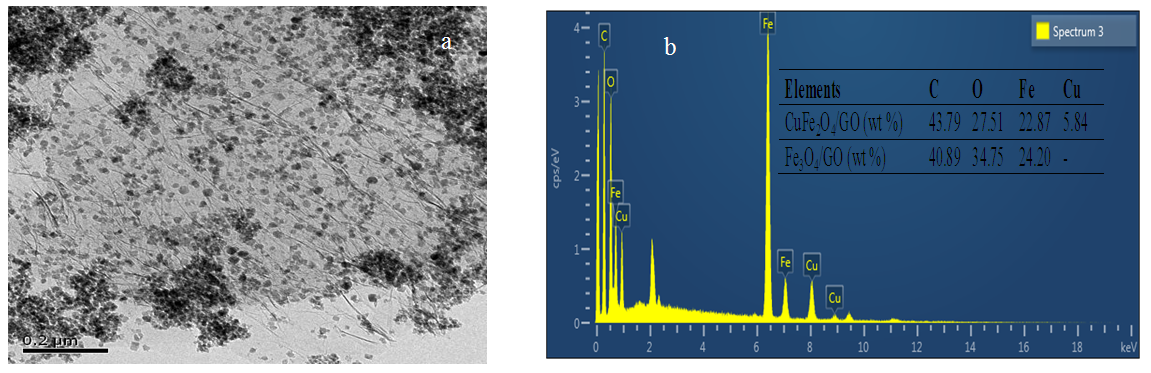 | Figure 2. TEM images (a) and EDX spectra (b) of CuFe2O4/GO |
3.3. Magnetization of CuFe2O4/GO
- Fig. 3 shows the magnetization hysteresis loop of the the CuFe2O4/GO nanostructures. The magnetic hysteresis loops were Slike curves. The results showed no remanence or coercivity in the hybrids suggesting that there was no remaining magnetization when external magnetic field was removed and the magnetic composites were superparamagnetic. The saturated magnetization of the superparamagnetic was nearly 35 emu/g which guaranteed catalyst separation by applying magnetic field out side.
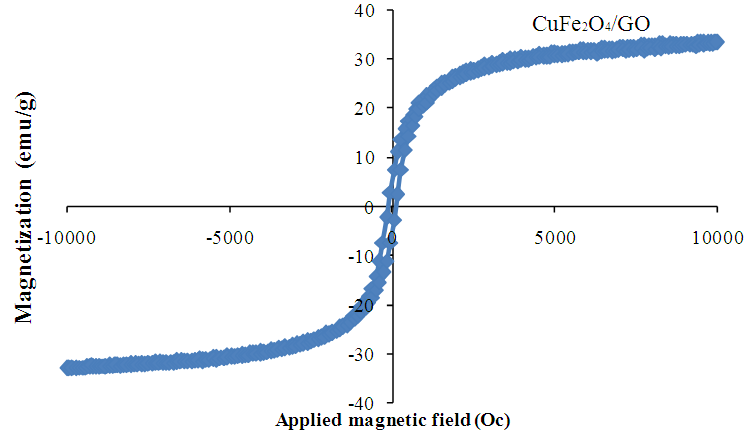 | Figure 3. Magnetization curve of CuFe2O4/GO |
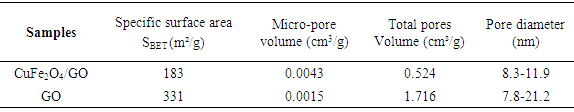
3.4. N2 Adsorption–Desorption Isotherms (BET) Analysis
- N2 adsorption–desorption isotherms of CuFe2O4/GO composites and GO are shown in figure 4. The N2 adsorption-desorption curve of CuFe2O4/GO composites displayed type IV with hysteresis corresponding to the capillary condensation which is typical for mesoporous material [16]. Thus, mesopore volume of composite reach 99% of total pore volume (0.524 cm3/g). This indicates that in the pore distribution, micropore volume is only minor. The pore texture parameters and surface area were listed in table 1. CuFe2O4/GO composites and GO had pore diameter of 8.8 to 21 nm and surface area of 183 m2/g and 331 m2/g, respectively. Note that GO has a main contribution of mesopore volume lager surface. CuFe2O4/GO particles were well dispersed on GO surface and consequently particles size is smaller (i.e surface area of particles is higher) due to the suppressing particles aggregation. However, the deposition and intercalation of CuFe2O4 particles within GO layers caused the decrease of surface are and pore volume as compared to that of the pure GO.
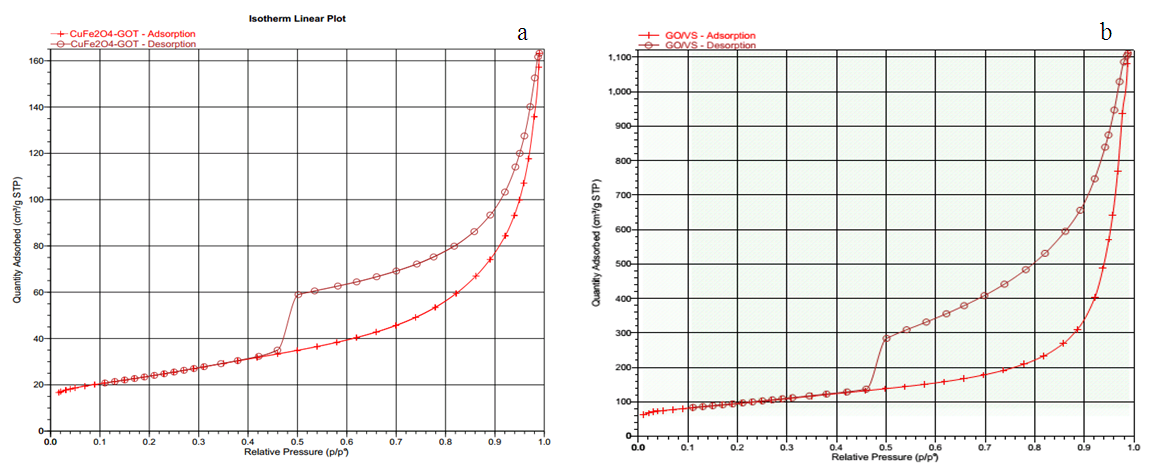 | Figure 4. N2 adsorption – desorption isotherms of CuFe2O4/GO (a) and GO (b) |
|
3.5. The X-ray Photoelectron Spectroscopy (XPS) Analysis
- XPS spectra of CuFe2O4/GO were show in fig. 5.The widely scan of the CuFe2O4/GO showed photoelectron lines at binding energy of 285 eV, 530 eV, and 711 eV correspond to the C1s, O1s, and Fe2p, respectively (Fig. 5). The deconvolution of the C1s peak was consisted of three peaks at 284.4 eV, 285.6 eV and 288.3 eV, which were ascribed to the C-C, C-O, and C(O)O in GO sheets. In the case of XPS spectra of C1s, the peak appeared at 291 eV was assigned to the π-π* bond of rGO, GO [17]. Additionaly the peak at 291 eV is attributed to the bonding state between electron pairs and holes in GO and rGO structures [18]. The binding energy of Fe2p3/2 of the hybrid was located at 711 eV while the peak of Fe2p1/2 appeared at 725 eV. As obeserved in figure 5d, no peak at 932 eV and 941 eV which are characteristic for individual existence of Cu+ and Cu2+ indicated that no copper oxides were separately formed. The high-resolution XPS spectra of Cu2p and Fe2p (Figure 5c,d) indicated that oxidation state of Cu and Fe being Cu2+ and Fe3+, respectively in the CuFe2O4 nanocomposite [19].
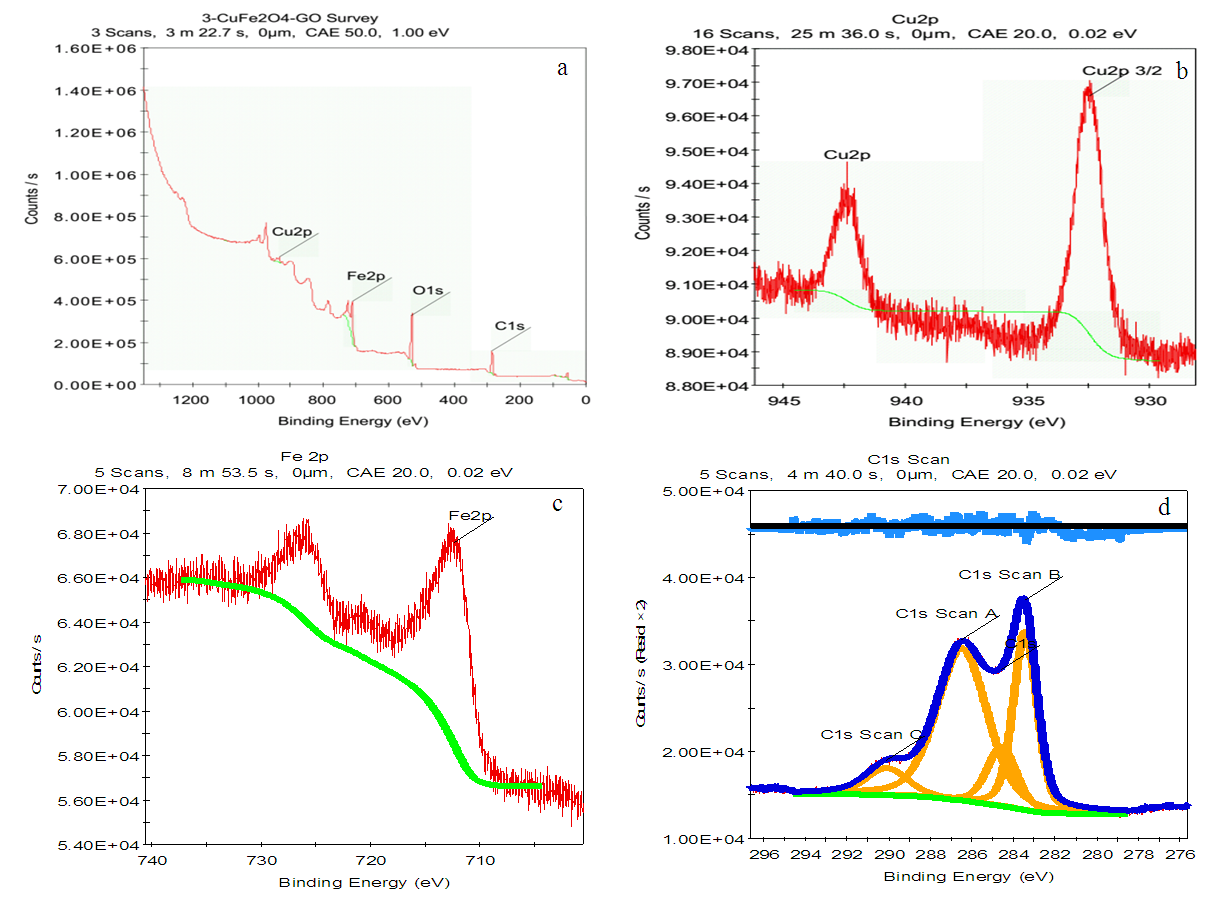 | Figure 5. XPS spectra of CuFe2O4/GO |
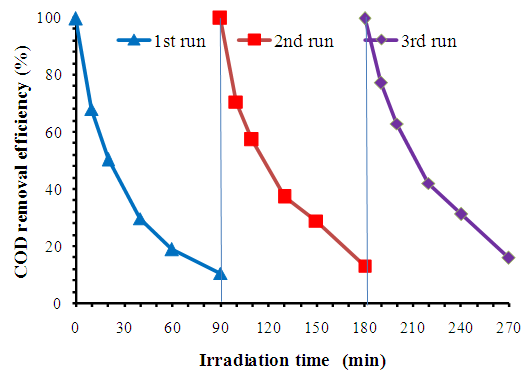
3.6. Photocatalytic Activity
- a/ Effect of catalyst dosageThe effect of different of catalyst concentrations on phenol degradation CuFe2O4/GO is shown in Fig. 6. The removal efficiency increases from 50.6% to 90.4% with increasing of CuFe2O4/GO concentration from 0.05 g/L to 0.3 g/L. However, no significant difference in removal of phenol by further increasing concentration of CuFe2O4/GO from 0.1 to 0.3 g/L was observed. As expected, the amount of active sites on CuFe2O4/GO rose with increasing dosage of catalyst, which promoted the generation of radicals, and the higher reaction rate is achieved. This can be explained that OH. radicals decomposed more rapidly, but the reaction occurred not rapid enough to consume the OH. radicals generated [9]. Therefore, the use of higher catalyst concentration of 0.1 g/L is not necessary.
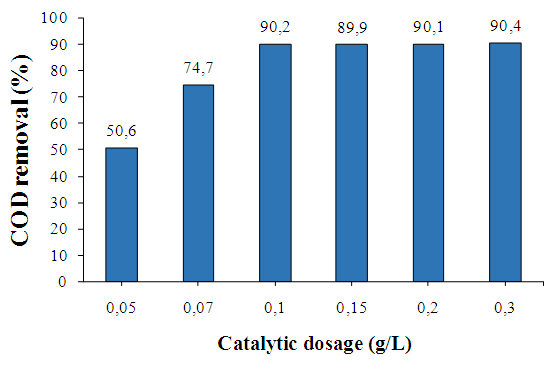 | Figure 6. Effect of catalyst concentration on degradation of phenol, [Phenol] = 100 mg/L, [H2O2]= 136 mg/L pH = 3; T = 303 K |
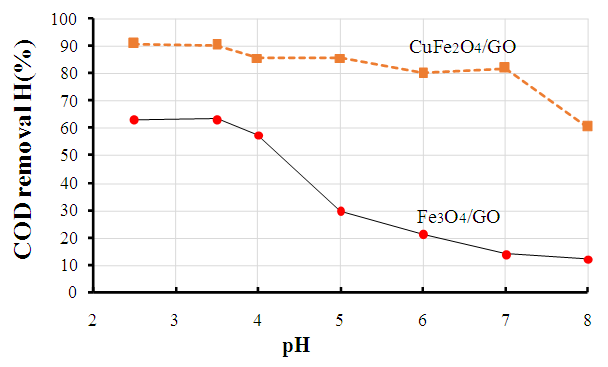 | Figure 7. Influence of pH values on phenol degradation over Fe3O4/GO and CuFe2O4/GO composites |
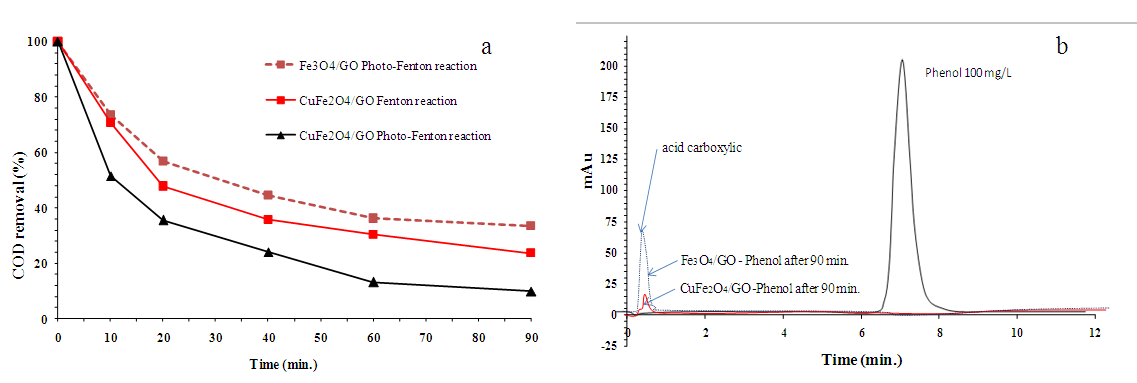 | Figure 8. (a) Comparison of the photocatalytic degradation of phenol solution under different conditions. (b) HPLC diagram of phenol degradation over CuFe2O4/GO |
4. Conclusions
- Ÿ CuFe2O4/GO composite was successfully synthesized by co-precipitation in “situ” using FeCl3.6H2O and CuCl2.2H2O as iron and copper sources on GO and followed by thermal reduction. From the XRD, XPS results, it revealed the formation of spinel CuFe2O4 on GO surface. From TEM images, it showed that CuFe2O4 composite has particle size of 20-30 nm and uniform size distribution.Ÿ CuFe2O4/GO composite exhibited high photo fenton activity (COD removal 90% after 90 min. reaction). The high catalytic activity of present nano CuFe2O4 particles can be ascribed to high catalytic surface area (due to small sized CuFe2O4 and GO sheets), excellent synergistic coupling of Cu/Fe species and presence of graphene sheets which holds the organic species by π–π interactions. Additionally, this CuFe2O4/GO composite showed high magnetism so it can be easy recovered by applying the magnetic field out side.
ACKNOWLEDGMENTS
- The authors thanks the Institute of Chemistry and Vietnam Academy of Science and Technology -VAST for financial support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML