-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Physical Chemistry
p-ISSN: 2167-7042 e-ISSN: 2167-7069
2017; 7(1): 1-7
doi:10.5923/j.pc.20170701.01

Effect of Surfactants on the Corrosion Behavior of Aluminum in Acid Solutions Containing Chloride Ions
B. A. Abd-El-Naby1, O. A. Abdullatef2, W. A. El-Mahmody1
1Faculty of Science, Alexandria University, Alexandria, Alexandria, Egypt
2Faculty of Pharmacy, Pharos University, Alexandria, Egypt
Correspondence to: B. A. Abd-El-Naby, Faculty of Science, Alexandria University, Alexandria, Alexandria, Egypt.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The corrosion behavior of aluminium in 0.1 M HCl in presence of each of the cationic surfactant (Cetrimide) and the anionic surfactant Sodium Lauryl Sulphate (SLS) has been studied using mass loss method, potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) techniques. The results showed that Cetrimide acts as accelerator and SLS acts as inhibitor which confirms our previously suggested mechanism of the inhibition of the pitting corrosion of aluminium in acid solutions containing Cl- ions. The thermodynamics of the adsorption of SLS at the aluminium/solution interface and the effect of SLS on the kinetics of the dissolution reaction of aluminium in 0.1 M HCl have been also studied.
Keywords: Aluminium, Pitting corrosion, EIS, Polarization
Cite this paper: B. A. Abd-El-Naby, O. A. Abdullatef, W. A. El-Mahmody, Effect of Surfactants on the Corrosion Behavior of Aluminum in Acid Solutions Containing Chloride Ions, Physical Chemistry, Vol. 7 No. 1, 2017, pp. 1-7. doi: 10.5923/j.pc.20170701.01.
Article Outline
1. Introduction
- Aluminum and its alloys are widely used in many industries such as aluminum-air technology, food industry and desalination plants. These industrial applications are due to the low density, favorable mechanical properties, good finishing, benign effect on the environment and the human health. A glance at the electrochemical series indicates that the standard reduction potential of aluminum equals – 1.8 V, this leads to the conclusion that aluminum is a very active metal. However, aluminum possesses an excellent corrosion resistance due to the rapid formation of a coherent inert oxide layer (10-12 µm thick) and the passivity of aluminum has been also attributed to the formation of a chemisorbed layer of O2(g) on the surface of metal [1]. Aluminum is readily soluble in acid solutions containing Cl- ions and corrosion of aluminum by Cl- ions arises from damage to the protective oxide layer, and when the metal oxide film dissolves, the metal corrodes uniformly [2]. The process of local dissolution of the oxide film has been termed pitting [3], and attributed to the tendencies of the halides to form aluminum soluble complexes [4]. In general, pitting of aluminium can be controlled by the action of some inorganic [5-12] and organic additives [13-23] which retard the adsorption of Cl- ions and/or by the formation of more resistant oxide film on the metal surface [24].Our group of research is interested in the study of the dissolution of aluminum in acid solutions and its inhibition. Four categories of inhibitors were investigated in our laboratory using chemical and electrochemical techniques to clarify and discuss their inhibition characteristics and adsorption mechanisms. These four categories of inhibitors are: 1) simple and synthetic organic compounds [25], 2) inorganic additives [26-29], 3) Chelates [30], and 4) natural products respectively [31-33]. In our previous work [26-28] we looked into some thermodynamic and kinetic factors governing the pitting corrosion of aluminum and its inhibition. These studies proposed a mechanism of inhibition involving three competitive equilibria: 1) molecular oxygen adsorption, 2) Cl- ion adsorption and 3) inhibitor adsorption. This is followed by a rate determining Cl- ion catalyzed dissolution step of AlCl-ads.Due to the ability of the surfactants to adsorb on the surfaces, they should be effective corrosion inhibitors. The surfactant inhibitors have many advantages such as high inhibition efficiency, low price, low toxicity and easy production [34]. The inhibition characteristics of three novel synthesized amido-amine double tailed cationic surfactants [35] and Cetyltrimethylammonium bromide surfactant [36] have been investigated recently in our laboratory. Mass loss, potentiodynamic polarization and electrochemical impedance spectroscopy techniques were used to determine the efficiency of these surfactants in the inhibition of the acidic corrosion of steel. Thermodynamics of adsorption of these surfactants and their effects on the kinetics of the dissolution reaction of steel in acid solutions have been discussed.Bronzoi et al, [37] studied the inhibition of the corrosion of pure aluminum in 0.5 M HCl by Tween 20, Tween 81 and hexadecylpyridinum bromide (HDPB) surfactants using the potentiodynamic polarization technique. The results showed that Tween 20 and 81 are more effective to inhibit the corrosion of pure aluminium than HDPB. The inhibition process was attributed to the formation of adsorbed film on the metal surface that protects the metal against corrosion species. Zhao et al [38] investigated the adsorption and inhibition characteristics of dodecyl benzene sulfonic acid sodium salt (DBSASS) and sodium dodecyl sulphate (SDS) anion surfactants for the corrosion of aluminum in 1.0 M HCl using weight loss method. It has been found that Langumir adsorption isotherm fit the experimental data. The thermodynamic parameters such as adsorption heat, adsorption entropy and adsorption free energy were calculated.The aim of this work is to study the effect of an anionic surfactant (Sodium Lauryl Sulfate, SLS) and a cationic surfactant (Cetyltrimethylammonium bromide, Cetrimide) on the pitting corrosion of aluminum in 0.1 M HCl, using weight loss, potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) techniques. It is important in this study to confirm the previous proposed mechanism of the inhibition of pitting corrosion of aluminum and discuss the adsorption processes of the surfactants at the aluminium/solution interface.
2. Experimental
2.1. Electrochemical Measurements
- A frequency response analyzer potentiostat (ACM 604) was used to carry out the electrochemical impedance and polarization measurements. The frequency range for EIS measurements was 1 x 104 to 0.1 Hz with applied potential signal amplitude of 10 mV around the rest potential. A three electrode made cell contains an auxillary graphite electrode and saturated calomel reference electrode was used. The working electrode was fabricated in cylindrical form. Aluminium was encapsulated in epoxy resin in such a way that only one surface was left uncovered. The working electrode has the chemical composition (% wt) Al 99.687; Mn 0.001; Zn 0.001; Ni 0.003; Fe 0.171; Si 0.135; Cu 0.001. The exposed area (0.5 cm2) sample was wet hand-polished using different grade emery papers of variable grades starting with a coarse one and proceeding in steps to the finest (1000) grade. The sample was then washed thoroughly with double-distilled water and finally dried by absolute ethanol just before immersion in the solution. Each experiment was carried out with newly polished electrode.Before polarization and EIS measurements, the working electrode was introduced into the test solution and left for 20 min at the open circuit potential. The polarization curve measurements were obtained at scan rate of 20 mV/min starting from cathodic potential (Ecorr -300 mV) going to anodic direction. All the measurements were done at 30.0 ± 0.1°C in solutions open to the atmosphere under unstirred conditions.To test the reliability and reproducibility of the measurements, duplicate experiments were performed in each case of the same conditions.
2.2. Weight Loss Measurements
- Rectangular specimens of aluminium with dimensions (2.0 cm x 5.0 cm x 0.05 cm) were used during weight loss measurements. The weight loss coupons were polished, cleaned and weighed, then suspended in beakers containing 100 ml of the test solutions. After definite time, the coupons were removed from the solution, washed with distilled water, ethanol and then dried by acetone and reweighed. The weight loss was then determined (gm cm-2hr-1), the experiment was then repeated for different time intervals up to 6 hours. To test the reliability and responsibility of the measurements, duplicate experiments were performed in each case of the same conditions.
2.3. Solution Preparation
- The test solutions were prepared from analytical grade reagents and distilled water. 36% HCl was purchased from Aldrich chemicals. Stock solution, of 1 M of HCl and 0.02 M of surfactant were used to prepare the test solution. Prior each experiment, 1.0 M HCl is added to an appropriate volume of 0.02 M surfactant solution and double distilled water to obtain a solution of 0.1 M HCl and the required concentration of the surfactant. Cetrimide and Sodium Lauryl Sulphate were obtained from Alpha Chemica with molecular weight 364.45 and 288.37g/mol respectively, their molecular structures are given in Figure 1.
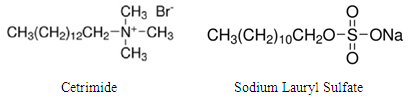 | Figure 1. Chemical structure of Cetrimide and Sodium Lauryl Sulfate |
3. Results and Discussion
3.1. Confirmation of the Previous Proposed Mechanism of the Inhibition of the Pitting Corrosion of Aluminum
- A general mechanism for the dissolution of aluminum in aqueous acidic solutions containing Cl- ions has been reported in the literature as follows [39]:
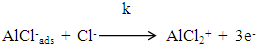
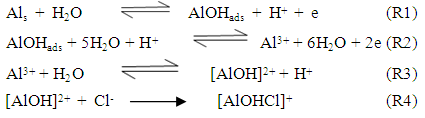 Zhang et al [40] suggested that the adsorption of the inhibitors on the aluminum surface partially displaces the water molecules originally adsorbed on the surface, which blocks the formation AlOHads [R2]. Thus both the oxidation reaction [R3] and the complexation reaction [R4] can be prevented.The following mechanism of the inhibition of the pitting corrosion of aluminum in acidic solutions containing Cl- ions has been proposed in our previous work [30]. This mechanism stated that in presence of an inhibitor and Cl- ion in the aerated acid solutions, three competitive equilibria are established: 1) molecular oxygen adsorption, 2) Cl- ion adsorption and 3) inhibitor adsorption. This is followed by a rate determining, chloride ion catalyzed dissolution step of AlCl-ads.
Zhang et al [40] suggested that the adsorption of the inhibitors on the aluminum surface partially displaces the water molecules originally adsorbed on the surface, which blocks the formation AlOHads [R2]. Thus both the oxidation reaction [R3] and the complexation reaction [R4] can be prevented.The following mechanism of the inhibition of the pitting corrosion of aluminum in acidic solutions containing Cl- ions has been proposed in our previous work [30]. This mechanism stated that in presence of an inhibitor and Cl- ion in the aerated acid solutions, three competitive equilibria are established: 1) molecular oxygen adsorption, 2) Cl- ion adsorption and 3) inhibitor adsorption. This is followed by a rate determining, chloride ion catalyzed dissolution step of AlCl-ads. 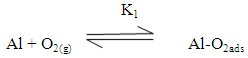 | (1) |
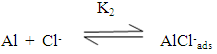 | (2) |
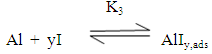 | (3) |
 | (4) |
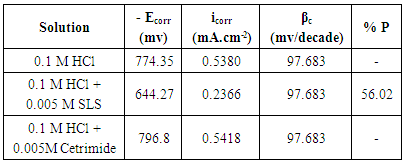
3.2. Potentiodynamic Polarization Results
- Figure 2 shows the polarization curves of aluminum in 0.1 M HCl solution in absence and presence of 0.005 M Cetrimide or SLS surfactants.
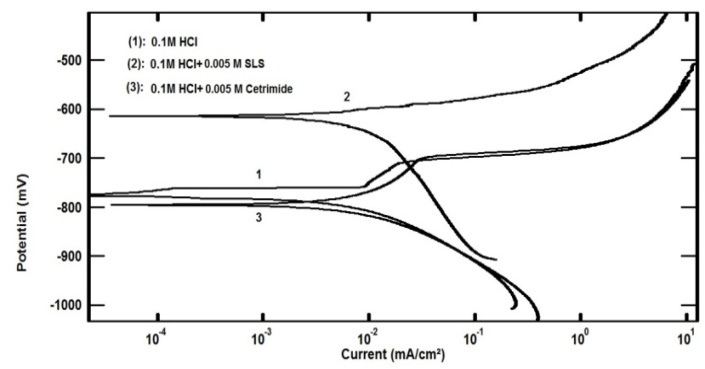 | Figure 2. Potentiodynamic polarization plots for aluminum in 0.1 M HCl solution in the absence and presence of 0.005 M Anionic and Cationic surfactants at 30°C |
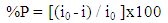 | (5) |
|
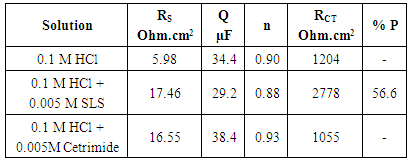
3.3. Electrochemical Impedance Spectroscopy (EIS) Results
- The Nyquist plots for aluminum in 0.1 M HCl in absence and presence of 0.005 M Cetrimide or SLS surfactants and the equivalent circuit model which fit well the impedance spectra are given in Figure 3(a,b). These plots manifested capacitive semicircle indicating that the dissolution of aluminum is mainly controlled by charge transfer process across the metal/solution interface. The equivalent circuit includes the solution resistance Rs shorted by the capacitor Cdl which is placed in parallel to the charge transfer resistance Rct.
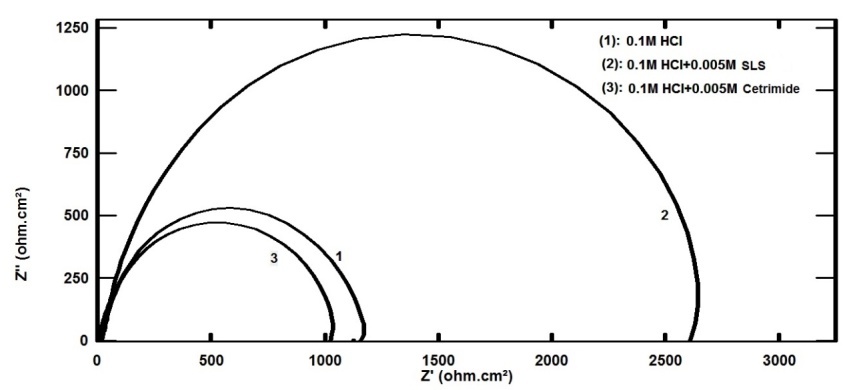 | Figure 3a. Nyquist plots for aluminum in 0.1 M HCl solution in the absence and presence of 0.005 M Anionic and Cationic surfactants at 30°C |
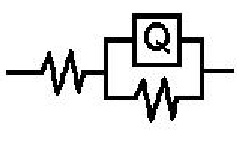 | Figure 3b. The equivalent circuit model |
|
3.4. Inhibition of Dissolution of Aluminium in 0.1 M HCl by Sodium Lauryl Sulfate (SLS)
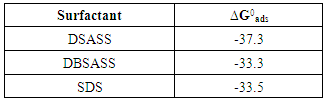
3.4.1. Thermodynamics of the Adsorption of SLS at the Aluminium/Solution Interface
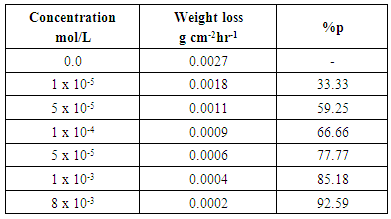
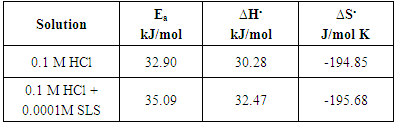
- Table 3. shows the results of the weight loss for dissolution of aluminium in 0.1 M HCl after 2 hours of immersion at 30°C. The percentage inhibition efficiency % P was calculated using the relation [35]:
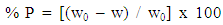 | (6) |
|
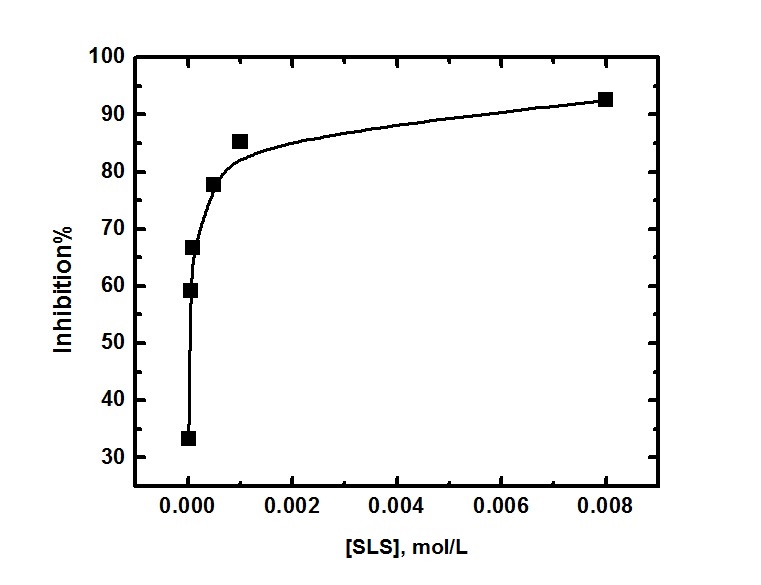 | Figure 4. The relation between the percentage inhibition and the concentration of SLS surfactant for aluminium in 0.1 M HCl |
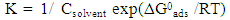 | (7) |
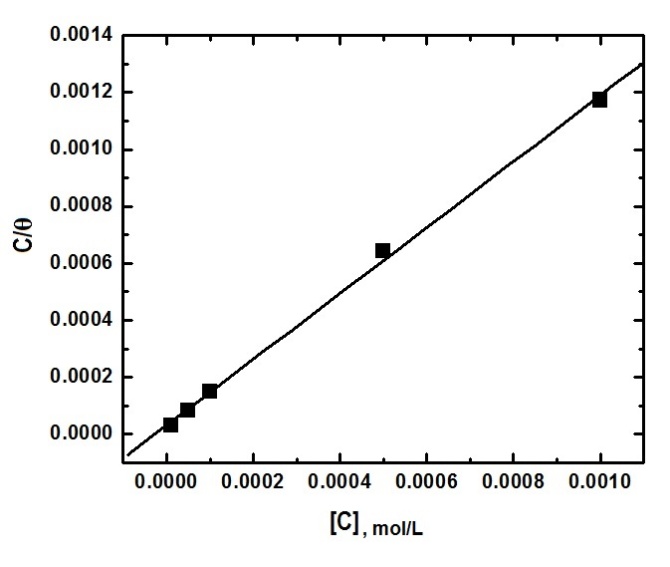 | Figure 5. Application of the Langmuir isotherm to fit the experimental results of adsorption of SLS on aluminium surface in 0.1 M HCl |
|
3.4.2. Effect of SLS on the Kinetics of the Dissolution Reaction of Aluminium in 0.1 M HCl
- The activation parameters of the dissolution reaction of aluminium in free acid solution and in presence of the surfactant were obtained from linear square fit of lnW and ln(W/T) versus (1/T) according to Arrhenius and Transition State equations [30].
 | (8) |
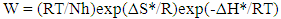 | (9) |
 | Figure 6a. Linear fit for (ln W) data vs. (1/T) for aluminium dissolution in 0.1 M HCl solutions in the absence and presence of 0.0001 M SLS |
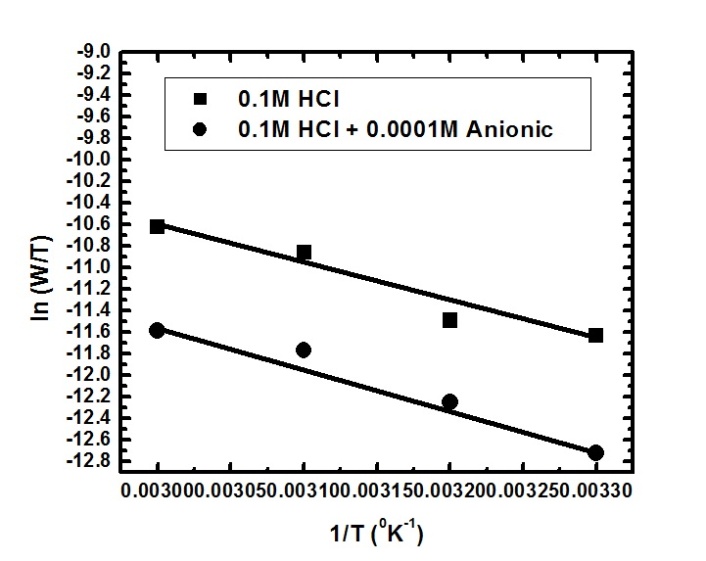
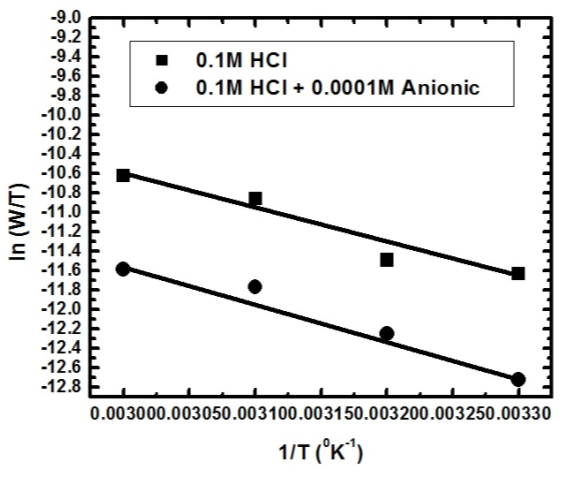 | Figure 6b. Linear fit for (ln W/T) data vs. (1/T) for aluminium dissolution in 0.1 M HCl solutions in the absence and presence of 0.0001 M SLS |
|
4. Conclusions
- 1) Potentiodynamic polarization and impedance results indicated that the cationic surfactant (Cetrimide) acts as accelerator of the pitting corrosion of alminium by Cl- ions, however, the anionic surfactant (SLS) prevent it.2) The results confirmed our previously suggested mechanism of the inhibition of the pitting corrosion of aluminium.3) The thermodynamic study of the adsorption of SLS at aluminium/solution interface indicated that the adsorption process is comprehensive (physical and chemical).4) The kinetic study of the dissolution reaction of aluminium in acidic solutions containing Cl- ions in presence of SLS indicated that the competitive adsorption of the surfactant anions increased the energy barrier of the corrosion reaction.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML