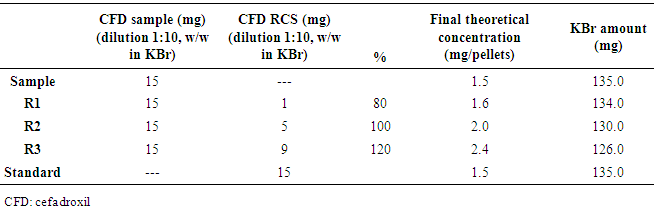
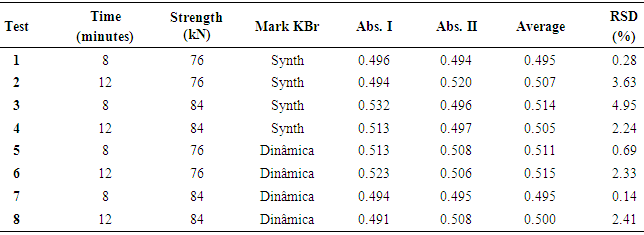
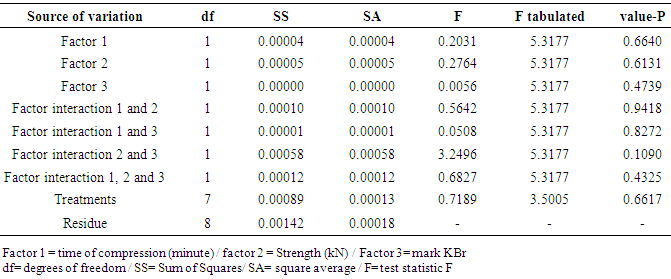
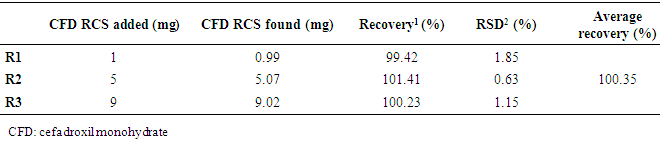
| [1] | ALY, F.A.; ALARFAFFJ, N.A.; ALWARTHAN, A.A. Permanganate-based chemiluminescence analysis of cefadroxil monohydrate in pharmaceutical samples and biological fluids using flow injection. Talanta, v.47, n.2, p. 471-478, 1998. |
| [2] | ANASTAS, P. Redesigning the material basis of four society and our economy. In: Green & Sustainable Chemistry Conference, 2016, Berlin. |
| [3] | ANDRASI, M.; BUGLYO, P.; ZEKANY, L.; GASPAR, A. A comparative study of capillary zone electrophoresis and pH-potentiometry for determination of dissociation constants. Journal of Pharmaceutical and Biomedical Analysis, v.44, p.1040-1047, 2007. |
| [4] | ARGENTINA Pharmacopoeia. 7. ed. Farmacopea Nacional Argentina, 2003. |
| [5] | AUDA, S.H.; MRESTANI, Y.; AHMED, AM.S.; NEUBERT, R.H.H. Characterization of the interaction of cefadroxil with different metal ions using CE. Electrophoresis, v.30, p.1066–1070, 2009. |
| [6] | BONFILIO, R.; ARAÚJO, M.B.; SALGADO, H.R.N. Recent applications of analytical techniques for quantitative pharmaceutical analysis: a review. WSEAS Transactions on Biology and Biomedicine, v.7, p.316-338, 2010. |
| [7] | BP. BRITISH Pharmacopoeia. London: Her Majesty’s Stationary Office, 2012. |
| [8] | BRAZIL. National Health Surveillance Agency (ANVISA). Resolution - RDC No. 899 of 29 May 2013. Guide for validation of analytical and bioanalytical methods. Official Gazette. Brasília, June 2, 2003. |
| [9] | BRAZIL. National Health Surveillance Agency (ANVISA). Resolution - RDC No. 17 of 16 April 2010. It has about good manufacturing practices medices. Official Gazette. Brasilia, August 21, 2006. |
| [10] | BRAZIL. National Health Surveillance Agency (ANVISA). List of reference medicines. http://www.anvisa.gov.br/medicamentos/referencia/lista.pdf. Accessed August 13, 2016a. |
| [11] | BRAZIL. Ministry of Health. List of generic drugs. http://www.anvisa.gov.br/hotsite/genericos/lista/registrados.htm. Accessed August 13, 2016b. |
| [12] | BRAZILIAN Pharmacopoeia. 5. ed. São Paulo: Atheneu, 2010. |
| [13] | CORRÊA, J.C.R.; SALGADO, H.R.N. A platform for designing quantitative infrared spectrophotometric method for drugs and pharmaceuticals analysis: a rediscover for an ecological and safer technique in the routine quality control laboratories. World Journal of Pharmacy and Pharmaceutical Sciences, v.3, n.6, p.2056-2059, 2014. |
| [14] | DEVALIYA, R.; JAIN, U. K. Noval estimation of cefadroxil in tablet dosage forms by RP-HPLC. Oriental Journal of Chemistry, v. 25, n. 4, p.1053-1058, 2009. |
| [15] | DEY, S.; KALYANI, K.; SAMYUKTHA, B.; SAHOO, S. K.; MOHAPATRA, S.; MURTHY, P. N.; KUMAR, D. Development and validation of a UV-Vis spectrophotometric method for the estimation and degradation monitoring of cefadroxil in bulk and pharmaceutical dosage forms. International Journal of Chemistry Research, v.1, n. 1, p. 29-34, 2010. |
| [16] | DHOKA, M.; CHOPADE, S. Method development & comparative statistical evaluation of HPLC & HPTLC method for simultaneous estimation of cefodrixil monohydrate & ambroxol hydrochloride. Indo Global Journal of Pharmaceutical Sciences, v.2, n.2, p.203-212, 2012. |
| [17] | EL-GINDY, A.; EL-WALILY, A.F.M.; BEDAIR, M.F. First-derivative spectrophotometric and LC determination of cefuroxime and cefadroxil in urine. Journal of Pharmaceutical and Biomedical Analysis, v.23, p.341-352, 2000. |
| [18] | EP. EUROPEAN Pharmacopoeia. 8. ed. Council of Europe, 2013. v.2. |
| [19] | FULIAS, A.; VLASE, T.; VLASE, G.; SZABADAI, Z.; RUSU, G.; BANDUR, G.; TITA, D.; DOCA, N. Thermoanalytical study of cefadroxil and its mixtures with different excipientes. Journal of Chimie, v.61, n.12, p.1202-1206, 2010. |
| [20] | GÁSPÁR, A.; ANDRÁSI, M.; KARDOS, S. A pplication of capillary zone electrophoresis to the analysis and to a stability study of cephalosporins. Journal of Chromatography B, v.775, p.239–246, 2002. |
| [21] | HANCU, G.; KELEMEN, H.; RUSU, A.; GYÉRESI, Á. Development of a capillary electrophoresis method for the simultaneous determination of cephalosporins. Journal of the Serbian Chemical Society, v.78, n.9, p.1413–1423, 2013. |
| [22] | HANCU, G.; SASEBEŞI, A.; RUSU, A.; KELEMEN, H.; CIURBA, A. Study of the electrophoretic behavior of cephalosporins by capillary zone electrophoresis. Advanced Pharmaceutical Bulletin, v.5, n.2, p.223-229, 2015. |
| [23] | HENDRIX, C.; ZHU, Y.; WIJSEN, C. ROETS, E.; HOOGMARTENS, J. Comparative study of liquid chromatographic methods for the determination of cefadroxil. Journal of Chromatography, v.634, p.257-261, 1993. |
| [24] | HERNÁNDEZ, M.; BORRULL, F.; CALULL, M. Analysis of antibiotics in biological samples by capillary electrophoresis. Trends in Analytical Chemistry, v.22, p.416-427, 2003. |
| [25] | HODGES, P. Green chemistry & the economy of the future. In: Green & Sustainable Chemistry Conference, 2016, Berlin. |
| [26] | ICH - International Harmonised Tripartite Guideline. Validation of analytical procedures: text and methodology Q2 (R1). Commission of the European Communities. Geneva, 2005. |
| [27] | IP. INDIAN Pharmacopoeia. 5. ed. Ghaziabad, Indian Pharmacopoeia Commission, 2007. |
| [28] | JAIN, M. S.; BAVASKAR, S. R.; BARHATE, S. D.; FEGADE, J. D. Simultaneous uv spectrophotometric methods for estimation of cefadroxil and probencid in tablet dosage form. Indian Journal of Pharmaceutical Science &Research, v. 4, n. 1, p. 18-21, 2014. |
| [29] | JP. JAPANESE Pharmacopoeia. 16th ed. Tokyo, Society of Japanese Pharmacopoeia, 2011. |
| [30] | KOGAWA, A.C.; SALGADO, H.R.N. Development and validation of infrared spectroscopy method for the determination of darunavir in tabets. Physical Chemistry, v.3, n.1, p.1-6, 2013. |
| [31] | KOGAWA, A.C.; AGUIAR, F. A.; GAITANI, C.M.; SALGADO, H. R.N. Validation of a stability indicating capillary electrophoresis method for the determination of darunavir in tablets and comparison with the of infrared absorption spectroscopic method. World Journal Pharmacy and Pharmaceutical Sciences, v.3, p.283-297, 2014. |
| [32] | KOGAWA, A.C.; MELLO, N. P.; SALGADO, H.R.N. Quantification of Doxycycline in Raw Material by an Eco-Friendly Method of Infrared Spectroscopy. Pharmaceutica Analytica Acta, v.7, p. 463-466, 2016. |
| [33] | LI, Y.; VANDERGHINSTE, D.; PECANAC, D.; SCHEPDAEL, A.V.; ROETS, E.; HOOGMARTENS, J. Analysis of cefadroxil by micellar electrokinetic capillary chromatography: Development and validation. Electrophoresis, v.19, p.2890-2894, 1998. |
| [34] | LINDGREN, K. Determination of cefadroxil in serum by high-performance liquid chromatography with cephradine as internal standard. Journal of Chromatography, v.413, p.347-350, 1987. |
| [35] | LIU, H.; YU, A.; LIU, F.; SHI, Y.; HAN, L.; CHEN, Y. Chiral separation of cefadroxil by capillary electrochromatography, Journal of Pharmaceutical and Biomedical Analysis, v.41, p.1376–1379, 2006. |
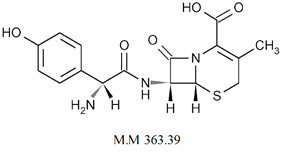
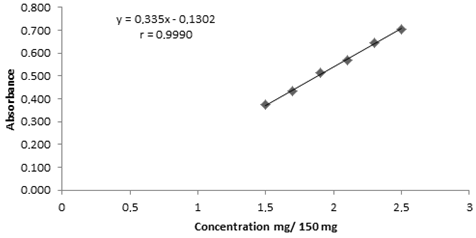
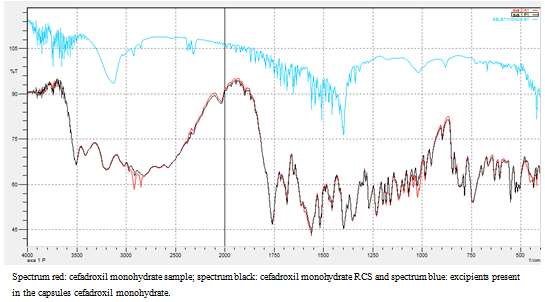
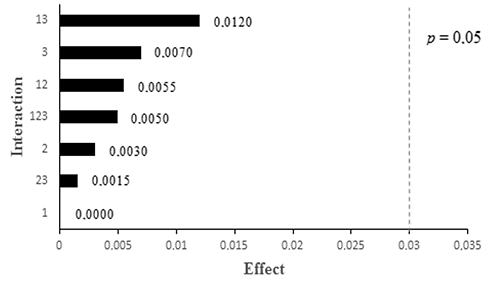
| [36] | MARCO, B.A.; SALGADO, H.R.N. Characteristics, properties and analytical methods of cefadroxil: a review. Critical Reviews in Analytical Chemistry, 2016. DOI: 10.1080/10408347.2016.1219649. |
| [37] | MCATEER, J.A.; HLLTKE, M.F.; SIIBER, B.M.; FAULKNER, R.D. Liquid chromatographic determination of five orallyactive cephatosporins - cefixime, cefaclor, cefadroxil, cephalexin, and cephradine in human serum. Clinical Chemistry, v.33, n.10, p.1788-1790, 1987. |
| [38] | MORENO, A.H.; SALGADO, R.N. Development and validation of quantitative analysis of ceftazidime in powder for injection by infrared spectroscopy. Physical Chemistry, v.2, n.1, p.6-11, 2012. |
| [39] | MRESTANI, Y.; NEUBERT, R. H. H.; HÄRTL, A.; WOHLRAB, J. Determination of cephalosporins in urine and bile by capillary zone electrophoresis. Analytica Chimica Acta, v.349, p.207-213, 1997. |
| [40] | MRESTANIA, Y.; NEUBERT, R. H.H.; MUNKB, A.; WIESEB, M. Determination of dissociation constants of cephalosporins by capillary zone electrophoresis. Journal of Chromatography A, v.803, p.273–278, 1998. |
| [41] | NAGARAJAN, J. S. K.; VIMAL, C. S.; GEORGE, R.; DUBALA, A. Simultaneous pharmacokinetic assessment of cefadroxil and clavulanic acid in human plasma by LC–MS and its application to bioequivalence studies. Journal of Pharmaceutical Analysis, v.3, n. 4, p. 285-291, 2013. |
| [42] | NIES, A. Ethics, legislation and economics. In: Green & Sustainable Chemistry Conference, 2016, Berlin. |
| [43] | PAVIA, D.L.; LAMPMAN, G.M.; KRIZ, G.S.; VYVYAN, J.R. Introdução à espectroscopia. 4th ed. São Paulo: Cengage Learning, 2010. |
| [44] | PORTUGUESE Pharmacopoeia. 8. ed. Lisboa: Infarmed, 2007. |
| [45] | PRADIP, D.; SANTOSH, J.; SUMIT, G.; LAXMI, J. Development and validation of uv spectrophotometric estimation of cefadroxil in bulk and tablet dosage form using area under curve method. Indu Americam Journal of Pharmaceutical Sciences, v. 2, n. 2, p. 581-586, 2015. |
| [46] | RAO, K. G.; SHANKAR, B. U.; PHANINDRA, B.; NAIK, M. Development and validation of RP-HPLC method for the estimation of cefadroxil monohydrate in bulk and its tablet dosage form. Journal of Advanced Pharmacy Education & Research, v.4, n.1, p.71-74, 2014. |
| [47] | SAMANIDOU, V.F.; HAPESHI, E.A.; PAPADOYANNIS, I.N. Rapid and sensitive high-performance liquid chromatographic determination of four cephalosporin antibiotics in pharmaceuticals and body fluids. Journal of Chromatography, v.788, p.147-158, 2003. |
| [48] | SAMANIDOU, V.F.; IOANNOU, A.S.; PAPADOYANNIS, I.N. The use of a monolithic column to improve the simultaneous determination of four cephalosporin antibiotics in pharmaceuticals and body fluids by HPLC after solid phase extraction—a comparison with a conventional reversed-phase silica-based column. Journal of Chromatography, v.809, p.175-182, 2004. |
| [49] | SCHMIDT, A. H.; STEINER, M. S. UPLC-MS/MS in Support of Cleaning Validation Studies - Cephalosporin Antibiotics Production Facility. Git Laboratory Journal, v.19, 2012. |
| [50] | SHALAEVA, M.; KENSETH, J.; LOMBARDO, F.; BASTIN, A. Measurement of dissociation constants (pka values) of organic compounds by multiplexed capillary electrophoresis using aqueous and cosolvent buffers. Journal of Pharmaceutical Sciences, v.97, n.7, p.2581-2606, 2008. |
| [51] | SHARIF, S. I.; KHAN, U; ASHFAQ, M.; IQBAL, M. S.; AHMAD, S. Development and validation of high performance liquid chromatographic method for the simultaneous determination of potassium clavulanate and cefadroxil in synthetically prepared tablets. Journal of Analytical Chemistry, v. 65, p. 1053-1058, 2010. |
| [52] | SOLANGI, A.R.; MEMON, S.Q.; KHUHAWAR, M.Y.; BHANGER, M.I. Quantitative analysis of eight cephalosporin antibiotics in pharmaceutical products and urine by capillary zone electrophoresis. Acta Chromatographica, n.19, p.81-96, 2007. |
| [53] | SUN, Y.; TANG, Y.; YAO, H.; ZHENG, X. Potassium permanganate–glyoxal chemiluminescence system for flow injection analysis of cephalosporin antibiotics: cefalexin, cefadroxil, and cefazolin sodium in pharmaceutical preparations. Talanta, v.64, n.1, p.156-159, 2004. |
| [54] | TANRISEVER, B.; SANTELLA P. J. Cefadroxil. A review of its antibacterial, pharmacokinetic and therapeutic properties in comparison with cephalexin and cephradine. Drugs, v.32, p.1-16, 1986. |
| [55] | THONGPOON, C.; LIAWRUANGRATH, B.; LIAWRUANGRATH, S.; WHEATLEY, R.A.; TOWNSHEND, A. Flow injection chemiluminescence determination of cefadroxil using potassium permanganate and formaldehyde system. Journal of Pharmaceutical and Biomedical Analysis, v.42, n.2, p.277-282, 2006. |
| [56] | TÓTOLI, E.G.; SALGADO, H.R.N. Development and validation of the quantitative analysis of ampicillin sodium in powder for injection by Fourier-transform infrared spectroscopy (FT-IR). Physical Chemistry, v.2, n.6, p.103-108, 2012. |
| [57] | USP 37. The United States Pharmacopeia. 37. ed. Rockville: The United States Pharmacopeial Convention, 2014. |
| [58] | VIEIRA, D.C.M.; RICARTE, P.C.; SALGADO, H.R.N. Development and validation of the quantitative analysis of cefuroxime sodium in powder for injection by infrared spectroscopy. Advances in Analytical Chemistry, v.2, n.6, p.80-87, 2012. |



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML