-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Physical Chemistry
p-ISSN: 2167-7042 e-ISSN: 2167-7069
2016; 6(3): 57-66
doi:10.5923/j.pc.20160603.01

Green Inhibitors for the Acidic Corrosion of Steel
B. A. Abd-El-Nabey1, S. El-Housseiny1, G. A. El-Naggar2, E. A. Matter2, G. Esmail2
1Alexandria University, Faculty of Science, Chemistry Department, Ibrahimia, Alexandria, Egypt
2Damanhur University, Faculty of Science, Chemistry Department, Damanhur, Egypt
Correspondence to: B. A. Abd-El-Nabey, Alexandria University, Faculty of Science, Chemistry Department, Ibrahimia, Alexandria, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The effects of Alhagi Maurorum, Morusnigra and Apricot leaves extracts on the corrosion of steel in aqueous 0.5M H2SO4 were investigated by weight loss, thermometric, potentiodynamic polarization, electrochemical impedance spectroscopy (EIS) techniques. The corrosion rate of steel was measured in absence and presence of different concentrations of the three extracts, and the percentage inhibition of the different solutions were determined using the four techniques. Effects of solvent of extraction on the inhibition efficiencies of the three extracts were discussed. Theoretical Kinetic-Thermodynamic model of adsorption of the inhibitors on the metal surface was tested to fit the experimental data of the three extracts. The activation parameters of the corrosion reaction of steel with H2SO4 in the absence and the presence of the three extracts were determined. The correlations between the thermodynamic and kinetics data and molecular structure of the chemical conistituents of the three extracts were discussed.
Keywords: Potentiodynamic Polarization, EIS, Weight Loss, Thermometric, Inhibitor, Steel, H2SO4
Cite this paper: B. A. Abd-El-Nabey, S. El-Housseiny, G. A. El-Naggar, E. A. Matter, G. Esmail, Green Inhibitors for the Acidic Corrosion of Steel, Physical Chemistry, Vol. 6 No. 3, 2016, pp. 57-66. doi: 10.5923/j.pc.20160603.01.
Article Outline
1. Introduction
- Acidic solutions are widely used in industry, where the most important fields of applications are acid pickling, acid cleaning, descaling and oilwell acidizing. Because of the general aggressiveness of acid solutions, inhibitors are commonly used to reduce the corrosive attack on metallic materials. Science 1985, our group of research is interesting in the study of the inhibition of the acidic corrosion of steel. There are four categories of inhibitors were investigated in our laboratory using the chemical and electrochemical techniques to clarify and discuss their inhibition characteristics and adsorption mechanisms. The first group of these inhibitors are simple and synthetic organic compounds such as amino acids [1], thiosemicarbazides [2-6], 4-amino-3-subestituted -5-hetero-1,2,4-triazolines [7], acid dihydrazides [8], 4-amino-3-hydrazino-5-thio 1,2,4-triazoles [9], nitriles[10] and dithiocarbamates [11].The second category of these inhibitors are chelating agents. Many authors focused on the use of chelating agents as possible corrosion inhibitors. Chelates are multi dentate ligands making available two or more donor atoms for binding. The idea is being good electron donors will enhance their adsorption on the surface of metal and increase their surface binding capabilities. Multi dentates know as macrocyclic ligands are a class of particular importance. The cyclic nature of these ligands through to enhance ligation and have stable complexes with metals of zero oxidation state. Several studies on the corrosion of steel in our laboratory have in fact shown the superior inhibition properties of chelates in general and of macrocyclic ligands in particular [12-14]. The third class of these inhibitors are the surfactants. The use of surfactants as corrosion inhibitors is one of the most practical methods for protection of metals against corrosion, especially in acidic media. Quaternary ammonium surfactants have source of uses because of their affinity for negatively charged surfaces and adsorption of these molecules depends mainly on certain physicochemical properties such as the presence of heteroatoms including oxygen, sulphur, nitrogen and phosphorus atoms and multiple bonds. This class of inhibitors has been also investigated in our laboratory [15-18]. The last category of these inhibitors are the plant extracts. The known hazard effects of most synthetic corrosion inhibitors are the motivation for the use of some natural products. Plant extracts are viewed as incredibly rich source of naturally acceptable, readily available and renewable source for a wide range of needed inhibitors. Extracts of chamomile, halffbar, black cumin, kidney bean, damssisa, lupine, olive leaves, nicotinya leaves and silene marmarica have been tested as inhibitors for acidic corrosion of steel in our laboratory and their inhibition mechanism have been discussed [19-24]. Recently, several authors studied the inhibition of the acidic corrosion of steel by the extracts of plant of Mourda Tinctoria [25], Bitter Leaf Root [26], Watermelon Rind [27], Musa Paradisica Peel [28], Nigella Sativa L. [29] and Brown Onion Peel [30].In this work, Alhagi Maurorum, Morusnigra and Apricot leaves extracts were investigated as corrosion inhibitors for steel in 0.5 M H2SO4 solutions using electrochemical impedance spectroscopy (EIS), potentiodynamic polarization techniques, weight loss and thermometric methods. The aim of the work is to: (a) test the credibility of the methods of determination of the corrosion rate, (b) explain the effect of the type of the solvent of extraction on the inhibition efficiency of the extract, (c) correlate the inhibition efficiency and the adsorption characteristics of the extracts with the chemical structure of their constituents.
2. Experimental
2.1. Electrochemical Measurements
- Electrochemical impedance and polarization measurements were achieved using frequency response analyzer (FRA)/potentiostat supplied from Parstate Instrument (PARSTAT 2263.02 SN 194). The frequency range for EIS measurements was 0.1 × 104 Hz with applied potential signal amplitude of 10 mV around the rest potential. The data were obtained in a three- electrode mode cell; graphite rod and saturated calomel electrodes (SCE) were used as counter and reference electrode. The material used for constructing the working electrode was steel that had the following chemical composition: (% wt) 0.21% C, 0.35% Si, 2.5% Mn, 0.04% P, and 0.04% S, rest Fe was used for the electrochemical corrosion studies in aqueous solutions. The working electrode was fabricated by cutting and shaping them in cylindrical forms. A long screw fastened to one end of the test cylinder for electrical connection. The Teflon gasket thereby forms a water-tight seal with the specimen electrode that prevents ingress of any electrolyte and thus avoiding crevice effect. The leak-proof assembly exposes only glass, only one side of rod was left uncovered as constant surface area in contact with the solution. The sample was wet hand-polished using different grade emery papers 320, 400, 600, 800 and 1000 grit finishes starting with a coarse one and proceeding in steps to the fine grit up to a mirror finish, washed thoroughly with double-distilled water and finally dried by absolute ethanol, just before immersion in the solution. Each experiment was carried out with newly polished electrode.Before polarization and EIS measurements, the working electrode was introduced into the test solution and left for 20 min to attain the open circuit potential (OCP) at which the change of OCP with time is 2 mV/min, i.e., the system had been stabilized. The polarization curve measurements were obtained at scan rate of 20mV/min starting from cathodic potential (Ecorr -300 mV) going to anodic direction. All the measurements were done at 30.0 ± 0.1°C in solutions open to the atmosphere under unstirred conditions.To test the reliability and reproducibility of the measurements, duplicate experiments were performed in each case of the same conditions.
2.2. Weight Loss Measurements
- Coupons with area 10 cm2 rectangular steel and with the same chemical composition of steel samples used in the electrochemical measurements were used in the experiment. The weight loss coupons were polished, cleaned and weighted, then suspended in beakers containing 100 ml of the test solutions. After definite time, the coupons were removed from the solution, washed with distilled water, ethanol and then dried by acetone and re weighted. The weight loss was then determined (g/cm2), the experiment was then repeated for different time intervals up to 6 hours. To test the reliability and responsibility of the measurements, duplicate experiments were performed in each case of the same conditions.
2.3. Thermometric Measurements
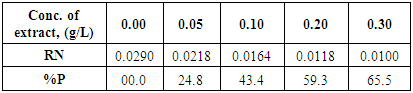
- The reaction vessel used in thermometry experiments was basically the same as described by Mylius [31]. The details of this method has been reported in our previous work [32]. The coupons of steel used in this method were similar to these used in the weight loss method. Exactly, 50ml of test solution was used for each experiment, the steel coupons was dipped in the solution in Mylius apparatus and the mercury reservoir of the used thermometer was rested on the steel specimen. From the rise in temperature of the system per minute, the reaction number (RN) was calculated using Equation 1 [32]
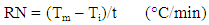 | (1) |
2.4. Solution Preparation
- The test solutions were prepared from analytical grade reagents and distilled water: 98% H2SO4 was purchased from Aldrich chemicals. The plant extracts were obtained by drying the plants for 1 h in an oven at 80°C and grinding to powdery form. A 10 g sample of the powder was refluxed in 100 mL double distilled water for 1 h. The refluxed solution was filtered to remove any contamination. The concentration of the plant extracts was determined by evaporating 10 mL of the filtrate and weighing the residue. Prior each experiment, 5M H2SO4 is added to an appropriate volume of the extract solution and double distilled water to obtain a solution of 0.5M H2SO4 and the required concentration of the extract.
2.5. Chemical Structure of the Constituents of the Extracts
- Figure 1 illustrates the chemical constituents of Alhagi Maurorum, Morusnigra and Apricot leaves extracts [33-35]. It is clear that the molecules of the constituents contains oxygen atoms and π-electrons bonds. Therefore, the adsorption at the metal/solution interface could take place via (i) dipole-type interaction between unshared electron pairs in the inhibitor with the metal, and (ii) the π-electrons bonds interaction with the metal [21].
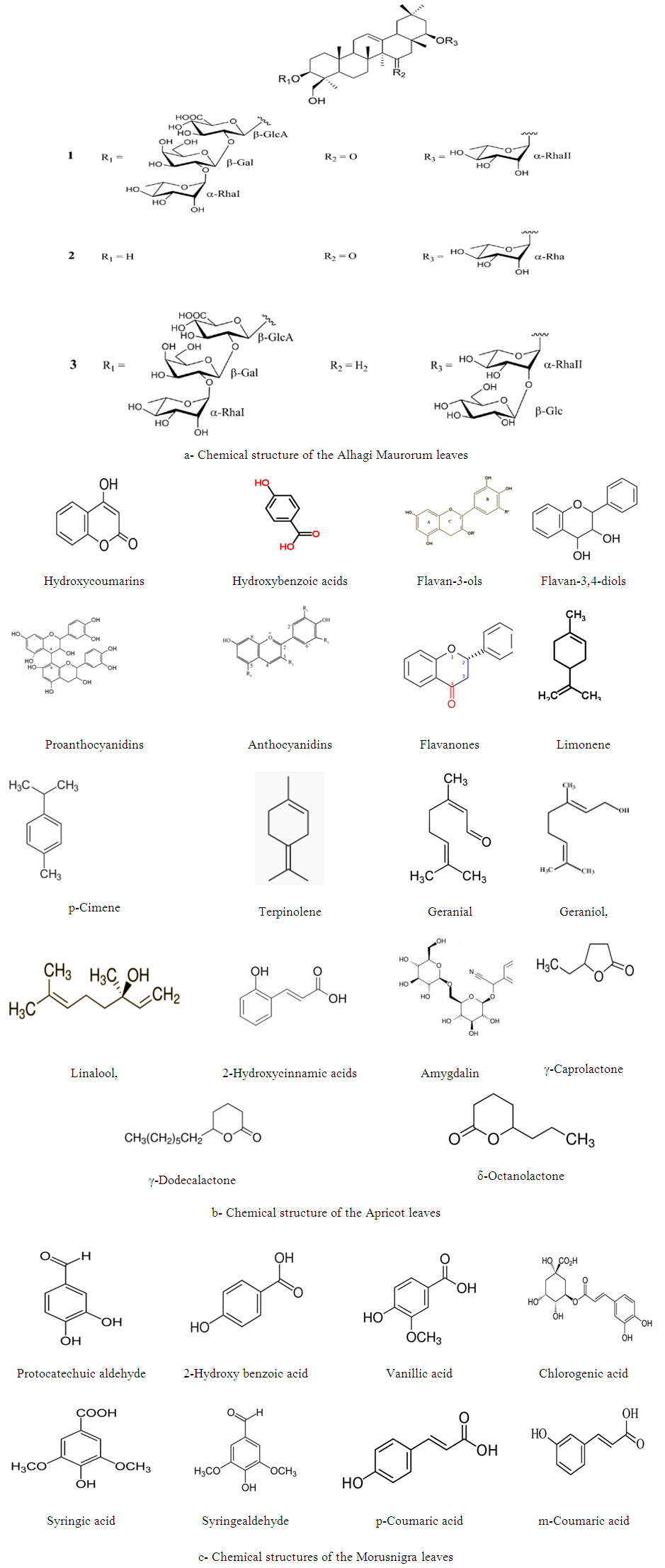 | Figure 1. Chemical structure of the constituents of the three extracts |
3. Results and Discussion
3.1. Credibility of the Methods of Determination of the Corrosion rate
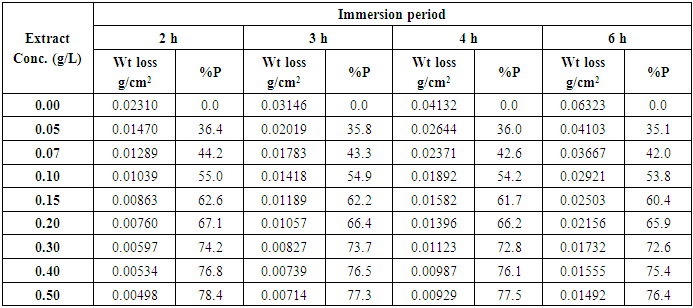
- Alhagi Maurorum leaves extract was investigated as inhibitor for the corrosion of steel in 0.5M H2SO4 using four methods of determination of the corrosion rate: weight loss, thermometric, potentiodynamic polarization and electrochemical impedance spectroscopy (EIS), in order to test the credibility of the four methods.a- Weight loss results Table 1 represents the mass loss results of steel in 0.5M H2SO4 in absence and presence of different concentrations of Alhagi Maurorum plant extract at different immersion times at 30°C. The percentage inhibition efficiency %P was calculated using the relation [18]:
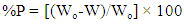 | (2) |
|
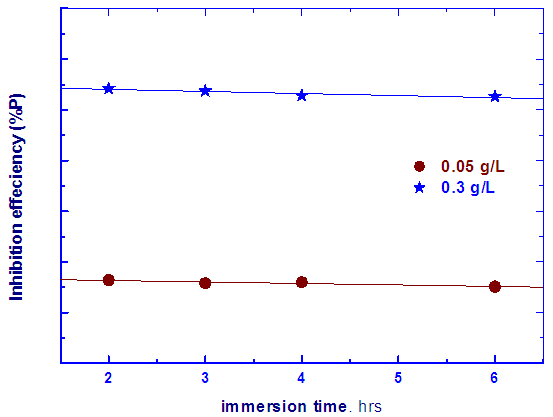 | Figure 2. Dependence of the percentage inhibition efficiency (%P) of two different solutions of Alhagi Maurorum leaves extract on the immersion time |
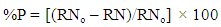 | (3) |
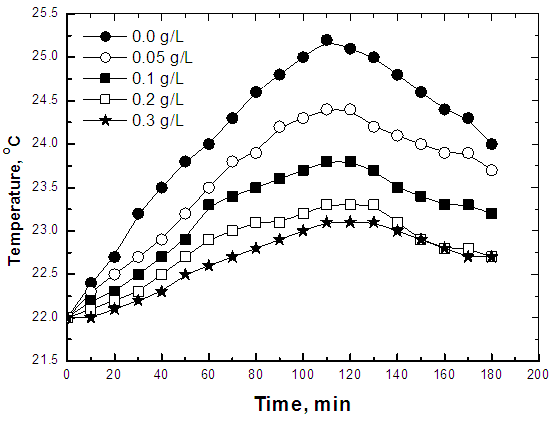 | Figure 3. Thermometric method of steel in 0.5 M H2SO4 solution in absence and presence of different concentrations of Alhagi Maurorum extract |
|
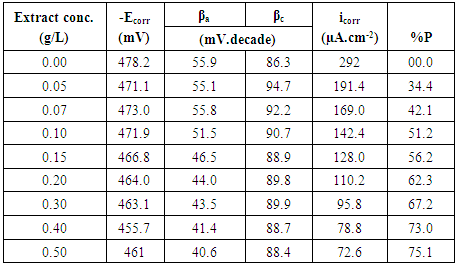
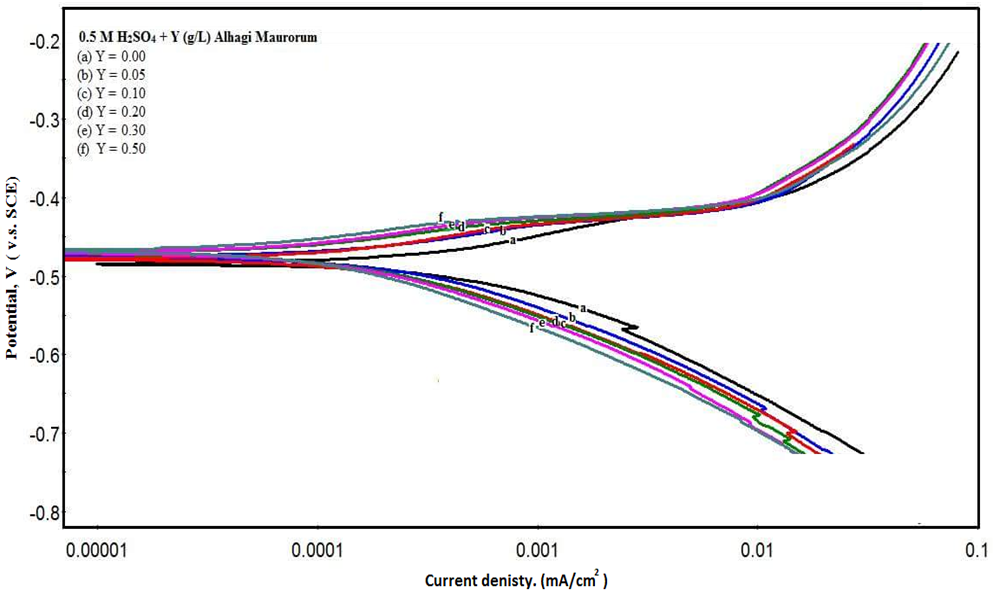 | Figure 4. Polarization curves of steel in 0.5 M H2SO4 solution in absence and presence of different concentrations of Alhagi Maurorum leaves extract |
|
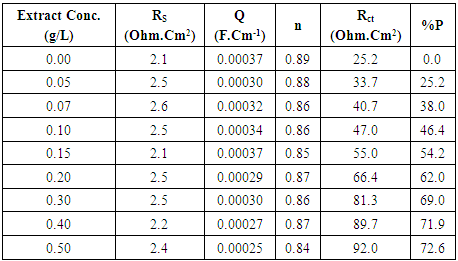
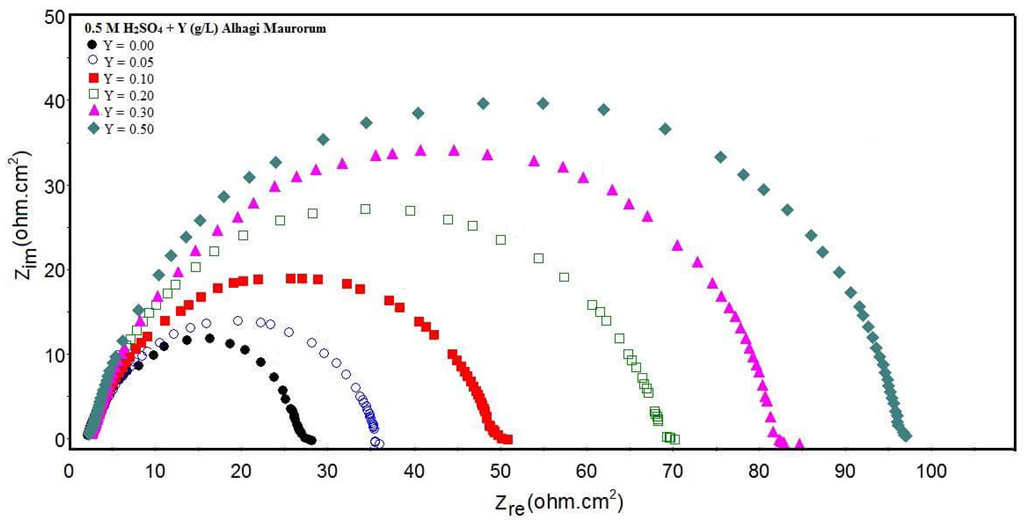 | Figure 5a. Nyquist plots for steel in 0.5 M H2SO4 solution in absence and presence of different concentration of Alhagi Maurorum leaves extract |
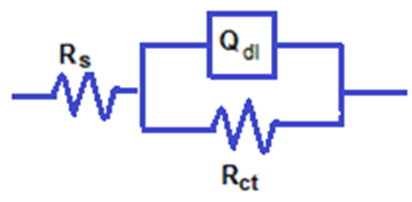 | Figure 5b. Schematic for the equivalent circuit of steel |
|
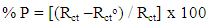 | (4) |
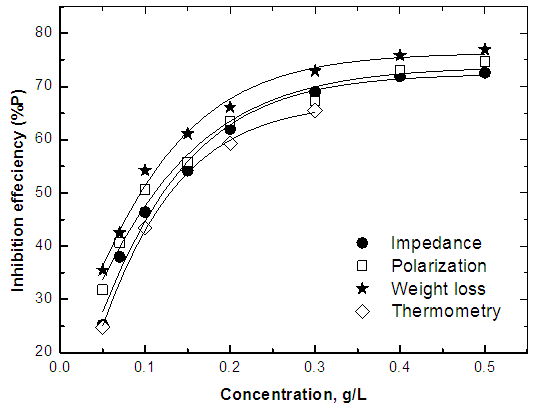 | Figure 6. Variation of percentage inhibition of the corrosion of steel in 0.5 M H2SO4 with the concentration of Alhagi Maurorum leaves extract using different techniques |
3.2. Correlation between the Chemical Structures of the Constituents of the Extract and Their Inhibition Efficiency
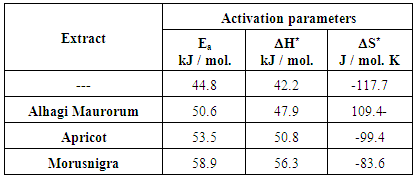
- Figure 7 shows the variation of the percent inhibition of the leaves extracts of each of Alhagi Maurorum, Morusnigra and Apricot with their concentrations. In order to investigate the correlation between the inhibition efficiency of these extracts and the chemical structure of their constituents, we will study: (a) the thermodynamics of the adsorption of the extracts at the steel/solution interface and (b) the effect of the extracts on the activation parameters of corrosion reaction between steel and H2SO4.
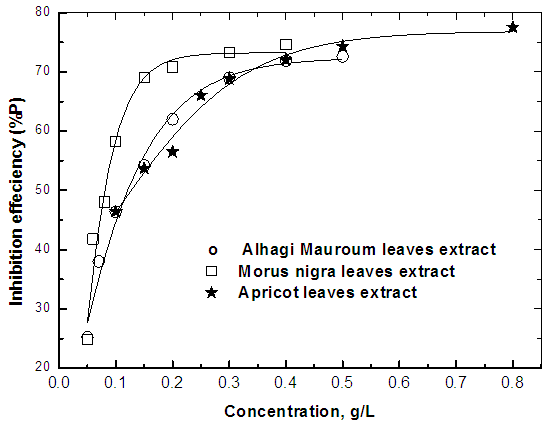 | Figure 7. Variation of percentage inhibition of the corrosion of steel in 0.5 M H2SO4 solution with the concentration of the three extracts |
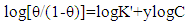 | (5) |
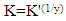 | (6) |
 | Figure 8. Application of the Kinetic-Thermodynamic model for the aqueous extracts of Alhagi Maurorum, Morusnigra and Apricot leaves |
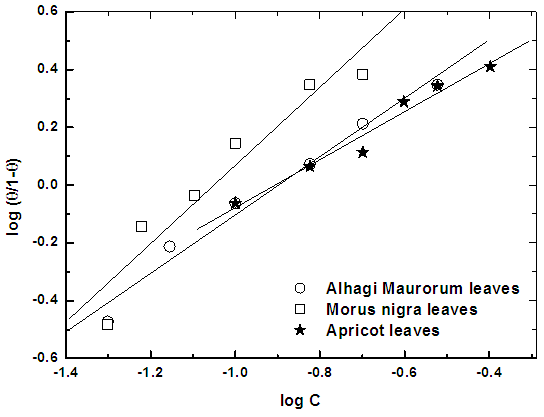
 | Figure 9. Linear fit for ln (W) data vs. (1/T) for steel dissolution in 0.5M H2SO4 in absence and presence of the three extracts |
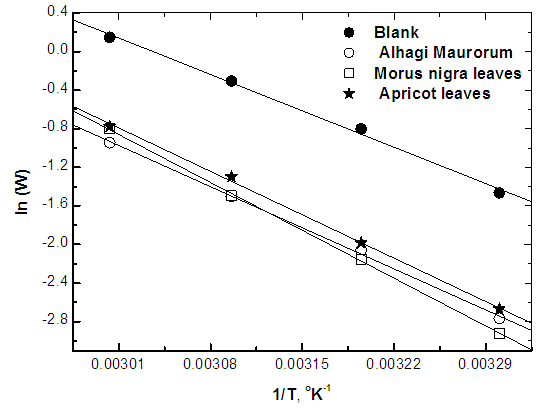
 | Figure 10. Linear fit for ln (W/T) data vs. (1/T) for steel dissolution in 0.5M H2SO4 in absence and presence of the three extracts |
|
3.3. Effect of the Nature of the Solvent of Extraction on the Inhibition Efficiency of the Extract
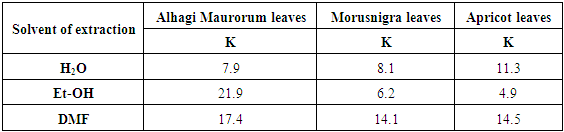
- Figure 11 shows the variation of the percent inhibition of the Alhagi Maurorum leave extract using water, ethanol or dimethyl formamide as solvent of extraction with its concentration, and figure 12 shows the application of the Kinetic-Thermodynamic model to the data of the Alhagi Maurorum leaves extract using water, ethanol or dimethyl formamide as solvent of extraction. It is clear that the Kinetic -Thermodynamic model is applicable to the data of the adsorption of Alhagi Maurorum leaves extract at the steel/solution interface. It has been found that the Kinetic -Thermodynamic model is applicable also for the experimental data of the adsorption of Morusnigra and Apricot leaves extracts at the steel/solution interface. Values of the binding constant “K” obtained for the three extracts are given in table 6. It is clear that Alhagi Maurorum leaves extract has the large value of K when the leaves are extracted by alcohol. However, K has the larger value for Morusnigra and Apricot leaves when extracted by dimethyl formamide. These results can be explained on the basis of the chemical structure of the constituents of the three plant leaves (Figure 1). It is clear from this figure that the chemical constituents of the extract of the leaves of Alhagi Maurorum consists mainly of saturated oxygen heterocyclic compounds, which are highly soluble in alcohol. However, in the case of Morusnigra and Apricot leaves extracts it is clear that their constituents contain mainly aromatic compounds which have high solubility in dimethyl formamide.
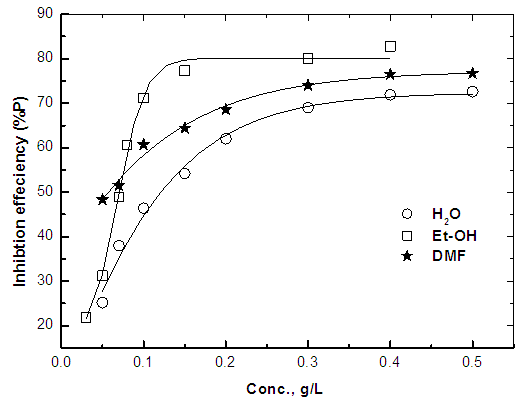 | Figure 11. Effect of the solvent of extraction on the variation of percentage inhibition of the corrosion of steel in 0.5 M H2SO4 with the concentration of Alhagi Maurorum leaves extract |
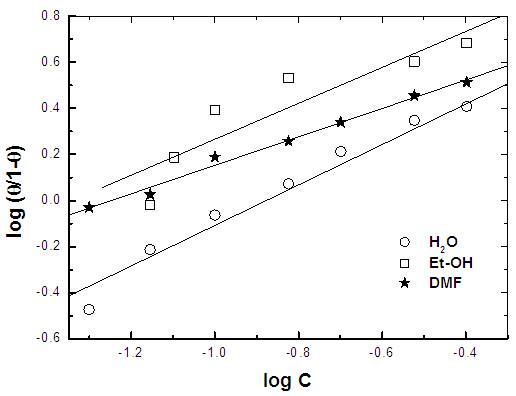 | Figure 12. Application of the Kinetic-thermodynamic model for the Alhagi Maurorum leaves extracted by different solvents |
4. Conclusions
- 1. The percentage of the inhibition efficiency of the leaves extracts of Alhagi Maurorum, Morusnigra and Apricot for the corrosion of steel in 0.5M H2SO4, obtained using the weight loss, thermometric, potentiodynamic polarization and EIS techniques indicated that there is a good agreement between the potentiodynamic and EIS results and also there is a fairly agreement between the result obtained from the electrochemical and chemical techniques.2. The application of the Kinetic-Thermodynamic model of the adsorption of the inhibitors at the metal /solution interface to the experimental data of the three extracts indicated that the binding constant “K” of the extracts is ordered in the form:Morusnigra leaves > Apricot leaves > Alhagi Maurorum leaves3. The effect of the extracts on the activation parameters of the dissolution of steel in 0.5M H2SO4 indicated that the increase in the values of each of Ea and ΔH* due to the presence of the extracts are in the same order given from thermodynamic data. 4. The order of increase of the values of K, Ea and ΔH* for the three extracts are discussed on the basis of the molecular structure of the chemical constituents of the extracts. 5. The efficiency of the inhibition of the three extracts for the corrosion of steel in 0.5M H2SO4 was found dependent on the solvent of extraction. This was discussed on the basis of the dependence of the solubility of the chemical constituent of the leaves of the plants in the solvents on the molecular structure of their constituents.
References
| [1] | B.A. Abd-El-Nabey, N. Khalil and A. Mohamed, Surf. Techn., 24 (1985) 383. |
| [2] | B.A. Abd-El-Nabey, E. Khamis, M. Shaban, G.E. Thompson and J.L. Ddwson, Surf. Techn., 28(1986)67. |
| [3] | B.A. Abd-El-Nabey, E. Khamis, G.E. Thompson and J.L. Dawson, Surf. Techn., 28 (1986)83. |
| [4] | B.A. Abd-El-Nabey, E. Khamis, G.E. Thompson and J.L. Dawson, Surf. Techn., 30 (1987)199. |
| [5] | E. Khamis and B.A. Abd-El-Nabey, Corros. Prev. Cont., 37(1990)127. |
| [6] | B.A. Abd-El-Nabey, A.M. Abdel-Gaber, G.Y. Elewady, M. M. El Sadeek, and H. M. Abd- El-Rahman., Int. J. Electrochem. Sci., 7(2012)11718. |
| [7] | B.A. Abd-El- Nabey, A. El-Toukhy, M. El-Gamal and F. Mahgoup, Surf. Techn., 27(1986)325. |
| [8] | B.A. Abd-El-Nabey, A.F. Hefny, E. Khamis, M.A. Khalifa and A.M. Michael, Steel Research, 59(1988)84. |
| [9] | A.B. Tadros and B.A. Abd-EI-Nabey, J. Electroanal. Chem., 24(1988)433. |
| [10] | A. Arab and B.A. Abd-El-Nabey, Int. J. Chem., 2(1991)23. |
| [11] | B.A. Abd-El-Nabey, A.A. El-Awady and S.G. Aziz, Corros. Prev. Contr., 38(1991) 68. |
| [12] | B.A. Abd-El- Nabey, M.A. Khalifa, E. Khamis, A.F. Hefny and A.R. Michael, Corros. Prev. cont., 35(1988)154. |
| [13] | A.A. El- Awady, B.A. Abd-El-Nabey, S.G. Aziz, M. Khalifa and R.A. Al-Ghamdey, Int. J. Chem., 1(1990)169. |
| [14] | A.A. El-Awady B.A. Abd-El-Nabey and S.G. Aziz, J. Electrochem. Soc., 139(1992)2149. |
| [15] | B.A. Abd El-Nabey, E. Khamis, M.Sh. Ramadan and A. El-Gindy, Corros., 52(1996)671. |
| [16] | B.A. Abd El-Nabey, S.A. El-Kharashi, M. Atteya, M.Sh. Ramadan and E. Khamis. J. Appl. Electrochem., 32(2002)775. |
| [17] | A. Abd-El-Nabey, O.A. Abdullatef, E. Khamis, W. A. El-Mahmody, Int. J. Electrochem. Sci., 11(2016)1271. |
| [18] | B.A. Abd-El-Nabey, H. A. Fetouh, I. Aiad , S. M. Shaban, S. El-Housseiny, and A. Maher, As. Int. J. Sci. & Tech., 7(2016)2491. |
| [19] | M. Abdel-Gaber, B. A. Abd-El Nabey, I.M. Sidahmed, A.M. El-Zayady and M. Saadawy, Corros., 62(2006)293. |
| [20] | A.M. Abdel-Gaber, B.A. Abd-El-Nabey, I.M. Sidahmed, A.M. El-Zayady, M. Saadawy, Corros. Sci., 48(2006)2765. |
| [21] | A.M. Abdel-Gaber, B.A. Abd-El-Nabey, M. Saadawy, Corros. Sci., 51(20091038. |
| [22] | A. M. Abdel-Gaber, B. A. Abd-El Nabey, E. Khamis and D.E. Abd Elkhalek., Desalination, 278(2011)337. |
| [23] | A.M. Abdel-Gaber, B.A. Abd-El-Nabey, E. Khamis, H. M. Abd- El-Rahman, H. Aglan, and A. Ludwick., Int. J. Electrochem. Sci., 7(2012)117930. |
| [24] | O.A. Abdullatef, B. A. Abd-El Nabey, Phys. Chem., 5(2015)39. |
| [25] | K. Krishnaveni, J. Ravichandran and A. Selvarary, Acta Metall. Sin., 26(2013)321. |
| [26] | I. Caroline Awe, A.S. Abdulrahman, H. Kobe Ibrahim, A.Ganiyu Kareem and S.M. Adams, Amer. J. Mater. Eng. Tech., 3(2015)35. |
| [27] | N.A. Odewunmi, S.A. Umoren and Z.M. Gasem, J. Indust. Eng. Chem., 21(2015)239. |
| [28] | G. Ji, S. Anjum, S. Sundaram and R. Parkash, Corros. Sci., 90(2015)107. |
| [29] | M. Chellouli, D. Chebabe, A. Dermay, H. Eramli, N. Bettach, N. Hajjaji, M.P. Casaletto, C. Cirrincione, A. Privitera and A. Svhiri, Electrochem. Acta, 204(2016)50. |
| [30] | K.C.R. Ferreira, R.F.B. Cordeira, J.C. Nunes, H. Orofino, M. Magalhaes, A.G. Torres and E.D Elia, Int. J. Electrochem. Sci., 11(2016)406. |
| [31] | M. Mylius, Z. Metallk, 14(1922)233. |
| [32] | B.A. Abd-El-Nabey, N. Khalil and E. Khamis, Corros. Sci., 25(1985)225. |
| [33] | B.A. Abd-El-Nabey, S. El-Housseiny, G.A. El-Naggar, E.A. Matter and G. Esmail Phys. Chem., 5(2015)49. |
| [34] | A. A. Memon, N. Memon, D. L. Luthria, M. I. Bhanger, A. A. Pitafi, Pol. J. Food Nutr. Sci., 60(2010)25. |
| [35] | I. E-Orhan, M. Kartal, Food Res. Int., 44(2011)1238. |
| [36] | K. Aramaki, Y. Node, and H. Nishihara, J. Electrochem. Soc., 137(1990)1354. |
| [37] | B.A. Abd-El-Nabey, A.M. Abdel-Gaber, M. El. Said Ali, E. Khamis, and S. El-Housseiny, Int. J. Electrochem. Sci., 7(2012)11811. |
| [38] | E.E. Foad El-Sherbini., S.M. Abdel Wahaab, and M. Deyab, Mater. Chem. and Phys., 89(2005)183. |
| [39] | G. K. Gomma, Mater. Chem. and Phys., 56(1998)27. |
| [40] | R. Guo, T. Liu, and X. Wei, Coll. and Surf. A: Physicochem. and Eng. Asp., 209(2002)37. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML