-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Physical Chemistry
p-ISSN: 2167-7042 e-ISSN: 2167-7069
2016; 6(1): 23-32
doi:10.5923/j.pc.20160601.03

Inhibitive Action of Sulphonic Acid on the Corrosion of Aluminium in NaOH Solutions
N. E. Ibisi , A. Iro
Department of Chemistry, College of Physical and Applied Sciences, Micheal Okpara University of Agriculture, Umudike, Nigeria
Correspondence to: N. E. Ibisi , Department of Chemistry, College of Physical and Applied Sciences, Micheal Okpara University of Agriculture, Umudike, Nigeria.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Corrosion inhibition of sulphonic acid on aluminium in 0.5M NaOH and 1M NaOH at 303K and 313K was studied using gravimetric technique. From the results obtained corrosion rate of aluminium reduced in the 0.5M NaOH and 1M NaOH solutions containing sulphonic acid from what was observed in the NaOH solutions without sulphonic acid. The inhibition efficiency increased as concentration of sulphonic acid increased. The corrosion inhibition of the inhibitor is attributed to its molecules getting adsorbed to the metal surface thereby creating a barrier between the metal surface and corrodent. The trend in thermodynamic parameters like activation energy and heat of adsorption and inhibition efficiency temperature relations, suggested that the inhibitor molecules were chemically adsorbed to the metal surface at 120minutes and below120minutes of the reaction period, but between 120minutes and 150minutes mixed adsorption of chemisorptions-physisorption mechanism was involved. The adsorption of the inhibitor fitted into Langmuir adsorption isotherm.
Keywords: Adsorption, Aluminium, Chemisorptions, Corrosion inhibition, Sulphonic Acid, Weight loss
Cite this paper: N. E. Ibisi , A. Iro , Inhibitive Action of Sulphonic Acid on the Corrosion of Aluminium in NaOH Solutions, Physical Chemistry, Vol. 6 No. 1, 2016, pp. 23-32. doi: 10.5923/j.pc.20160601.03.
Article Outline
1. Introduction
- There have been so many advancements in the world of science in recent years and corrosion has been one of the major areas of concentration due to its detrimental effect which is eating really deep into our metal world. Aluminium and its alloys are very attractive materials for engineering applications because of their lightweight and mechanical strength. Corrosion is the interaction between a metal or metal alloy and its environment. Many variables that influence the behavior of metals in their environment can result in accelerated corrosion. [1] The destructive nature of corrosion has been a major problem in science and technology, and preventive effort has attracted the attention of many scientists and researchers. This has led to the discovery of various experimental methods of preventing corrosion. Perry and Chilton [2] reported that the use of inhibitors was the most practical and cost effective means of checking corrosion. A corrosion inhibitor is a chemical compound that, when added in small concentration stops or slows down corrosion (rust) of metals and alloys. Corrosion inhibitor can be organic or inorganic substance, but the use of inorganic substance as corrosion inhibitor faces severe critics because of the toxic carcinogenic effect which poises danger to the environment. However the need to use environmental benign compounds leaves us with the only alternative of using organic compounds. [3], [4]. Inhibition of metal corrosion by organic compounds is as a result of adsorption of organic molecules or ions to the metal surface forming a protective layer. This layer reduces or prevents corrosion of the metal [5]. Some of the mechanisms involved in organic molecule corrosion inhibition include: (a) the formation of a passivation layer (a thin film on the surface of the material that stops access of the corrosive substance to the metal), (b) inhibiting either the oxidation or reduction part of the redox corrosion system (anodic and cathodic inhibitors), or (c) scavenging the dissolved oxygen.A study on other published literatures reveals the growing trend in the use of organic inhibitors of natural source because they are eco-friendly and sustainable. [6-8]. These organic inhibitors are composed of functional groups that contain hetero atoms like O, N, P, S etc which are reported in literatures to have corrosion inhibition properties. [4, 6, 15, 22], Based on this fact we suggest that sulphonic acid will be a good corrosion inhibitor in corrosive medium, since it contains S and O atoms (Lewis base) which have lone pair of electrons and the pi-electrons in the aromatic ring through which it can be adsorbed on the metal surface. The lateral interaction of the long chain carbon atoms due to Van der Waals forces can further facilitates formation of compact film of the inhibitor on the metal surface. [22] The current study investigates the inhibitive action of sulphonic acid on corrosion of aluminium in NaOH solution using gravimetric technique.
2. Materials and Methods
2.1. Materials Preparation and Solution
- The aluminium sheets were obtained from First Aluminium company in Port Harcourt with a percentage purity of 98%. The aluminium was mechanically press cut into coupons of dimension 3.0cm X 3.0cm and thickness of 0.045cm. These coupons were degreased in absolute ethanol and dried in acetone. The treated coupons were then stored in moisture free desiccators prior to use. The standard solution of the inhibitor (benzenesulphonic acid) of required concentrations of 50ppm, 100ppm, 150ppm and 200ppm were prepared by dissolving the appropriate weight of the inhibitor in 0.5M NaOH and 1M NaOH each, and respectively. The chemical formula for benzenesulphonic acid used is C6H6SO3H with structural formula shown in figure 1 below.
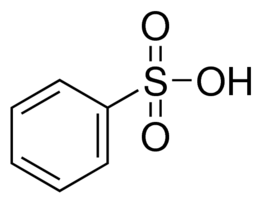 | Figure 1. Chemical structure of benezenesulphonic acid |
2.2. Weight Loss Measurement
- The aluminum coupons were weighed and stored in a moisture free desiccator. Each of the pre weighed aluminium coupon was removed from the desicaator and introduced into a 250ml beaker containing 100ml of 0.5M and 1M NaOH solution at room temperature. The stop clock was immediately started. After 30mins, the aluminium coupon was retrieved, washed thoroughly under running water, dried in acetone and the new weight determined and recorded. The procedure was repeated for time intervals of 60min, 90min, 120min and 150min using 0.5M and 1M NaOH solution respectively. The same procedure was employed for readings at 40°C. However, the beaker was kept in a thermostatic oven maintained at this temperature throughout the duration of the experiment. The weight loss ΔW was obtained by subtracting the weight after suspension from the initial weight; mathematically represented in equation 1
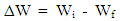 | (1) |
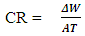 | (2) |
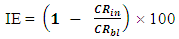 | (3) |
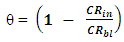 | (4) |
3. Results and Discussion
3.1. Gravimetric Studies
- The gravimetric analysis of aluminum in 1M NaOH and 0.5M NaOH solutions respectively, in the absence of inhibitor and in solutions containing different concentrations of sulphonic acid studied are represented in figure 2, 3, 4 and 5.
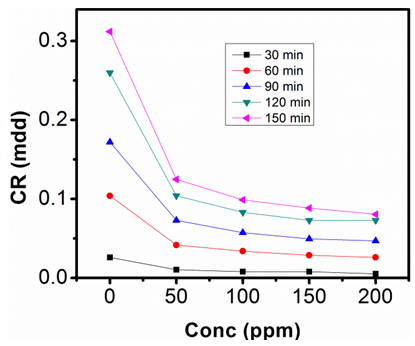 | Figure 2. Corrosion rate against Conc. of Sulphonic Acid in 0.5M NaOH at 303K |
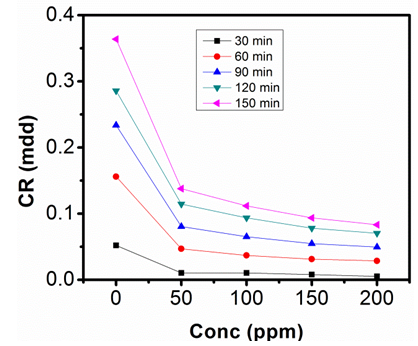 | Figure 3. Corrosion rate against Conc. of Sulphonic Acid in 0.5M NaOH at 313K |
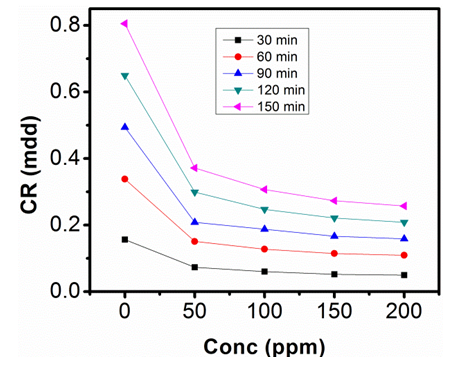 | Figure 4. Corrosion rate against Conc. of Sulphonic Acid in 1M NaOH at 303K |
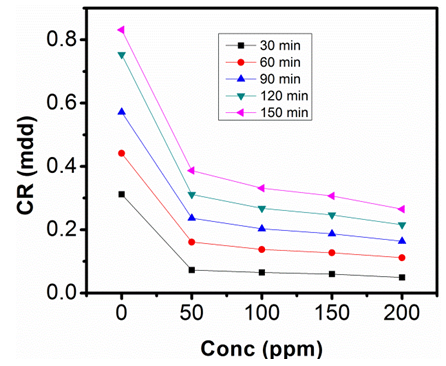 | Figure 5. Corrosion rate against Conc. of Sulphonic Acid in 1M NaOH at 313K |
3.2. Inhibition Efficiency
- The inhibition efficiencies calculated at each concentration of sulphonic acid studied are represented graphically in figures 9, 10, 11, and 12.Figure 6 is a graph of inhibition efficiency against concentration of sulphonic acid in 0.5M NaOH at 303K, figure 7 shows plot of inhibition efficiency against concentration of sulphonic acid in 0.5 NaOH at 313K. The graphs in figures 8 acid 9 are plots of inhibition efficiency against concentration of sulphonic acid in 1M NaOH at 303K and 313K respectively. Observations from the figures show increase in inhibition efficiency as the inhibitor concentration increases, both in 1M NaOH and 0.5M NaOH studied. The inhibition efficiency is observed to be more susceptible in 0.5M NaOH than what is observed in 1M NaOH.
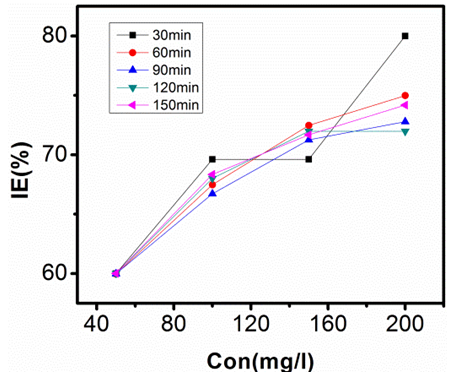 | Figure 6. Inhibition Efficiency against Conc. of Sulphonic Acid in 0.5M NaOH at 303K |
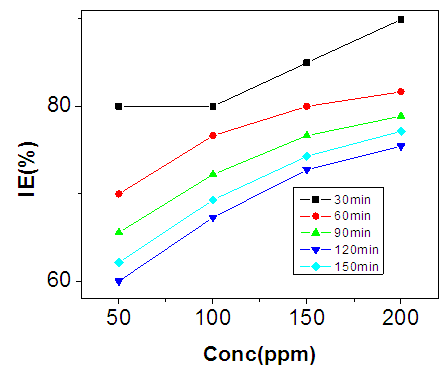 | Figure 7. Inhibition Efficiency against Conc. of Sulphonic Acid in 0.5M NaOH at 313K |
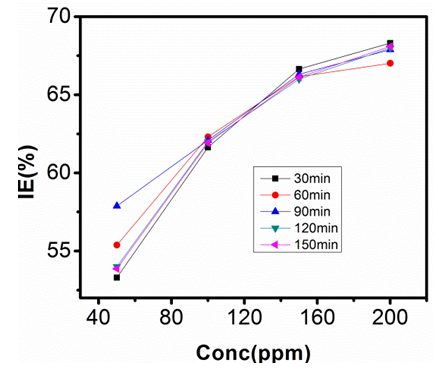 | Figure 8. Inhibition Efficiency against Conc. of Sulphonic Acid in 1M NaOH at 303K |
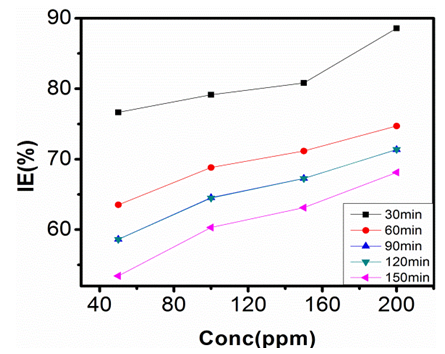 | Figure 9. Inhibition Efficiency against Conc. of Sulphonic Acid in 1M NaOH at 313K |
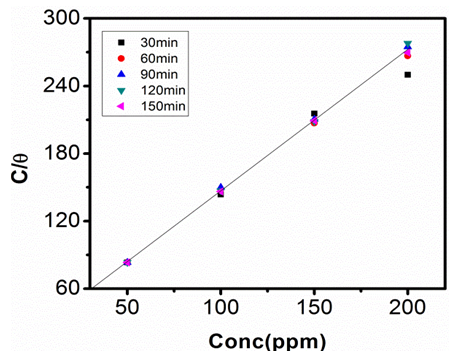 | Figure 10. Langmuir Adsorption Isotherm in 0.5M NaOH at 303K |
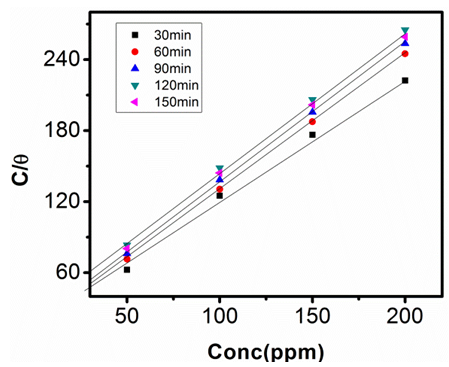 | Figure 11. Langmuir Adsorption Isotherm in 0.5M NaOH at 313K |
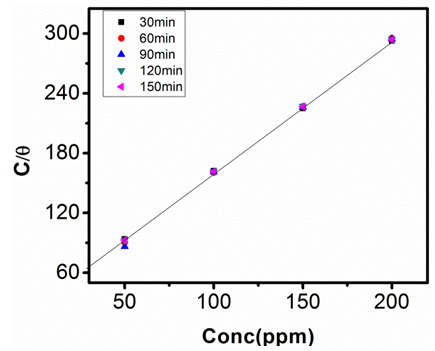 | Figure 12. Langmuir Adsorption Isotherm in 1M NaOH at 303K |
3.3. Adsorption Isotherm
- The relationship between the degree of surface coverage (θ) and sulphonic acid molecules can be represented by the Langmuir adsorption isotherm.
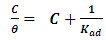 | (5) |
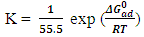 | (6) |
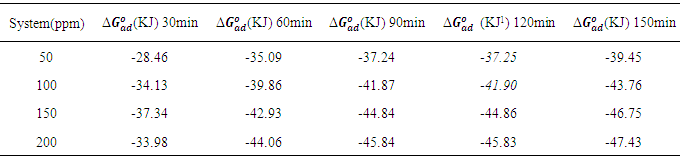
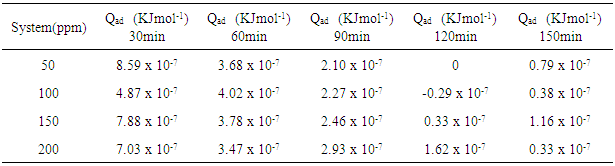
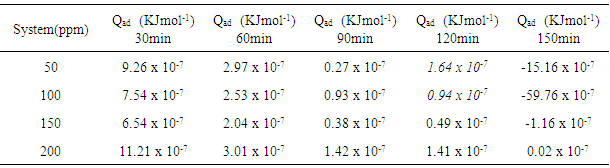
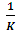 which suggests that the experiment fits into Langmuir adsorption isotherm.The calculated values of ΔG0ad are presented in tables 1, 2, 3, and 4. The negative signs on the values indicate spontaneous adsorption of sulphonic acid molecules to the surface of aluminum in corrosive medium. [4, 14, 15, 16, 17].
which suggests that the experiment fits into Langmuir adsorption isotherm.The calculated values of ΔG0ad are presented in tables 1, 2, 3, and 4. The negative signs on the values indicate spontaneous adsorption of sulphonic acid molecules to the surface of aluminum in corrosive medium. [4, 14, 15, 16, 17].
|
|
|
|
3.4. Effect of Temperature
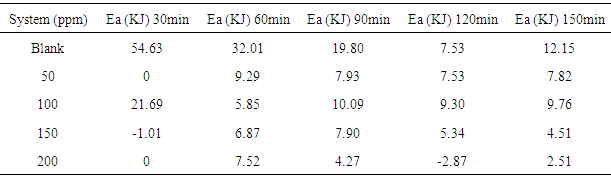
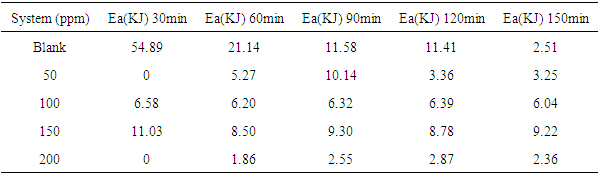
- Temperature has a significant influence on metal corrosion rates and inhibition efficiencies of corrosion inhibitors, when the corrosion reaction involves a cathodic process of hydrogen evolution, the corrosion rate increases exponentially with rise in temperature according to Arrhenius type dependence [13]. The inhibition efficiency relation gives insight to mechanism of adsorption of the inhibitor molecules to the surface of the metal, when the inhibition efficiency increases with rise in temperature the inhibitor molecules are said to be chemically adsorbed to the metal surface, but when it reduces with rise in temperature inhibitor molecules are said to be physically adsorbed to the metal surface. [21, 13, 14, 15, 17]. Figure 14 through figure 21 illustrates the effect of change in temperature on inhibition efficiency of sulphonic acid molecules on corrosion of aluminum in 0.5M NaOH and 1M NaOH. Figure 14 shows a plot of inhibition efficiency of 50ppm of sulphonic acid in 0.5M NaOH against temperature at various periods of immersion. If the graph is closely examined it can be observed that inhibition efficiency increased tremendously as temperature increases under immersion period of 30min, though the inhibition efficiency also increased as temperature increased under immersion periods of 60min, 90min, 120min and 150min, but the increase is not pronounced as observed from 30min of immersion. In fact the extent of increase reduces as the metal continues to stay in the solution, it can be seen that at 150min time of immersion the increase is almost not noticeable. Figure 15 contain a plot of inhibition efficiency of 100ppm of sulphonic acid in 0.5M NaOH against temperature for several immersion periods. It can be observed that inhibition efficiency increased as temperature increased accept at 120min and at 150min there was no observable change. In figure 16 a plot of inhibition efficiency of 150ppm of sulphonic acid in 0.5M NaOH is shown. It can be observed from the graph that inhibition efficiency increased as temperature rises, with a similar result in figure 14. Figure 17 is a plot of inhibition of efficiency of 200ppm of sulphonic acid against temperature studied. Results from the graph show increase in inhibition efficiency as temperature increases in all the period of immersion though the increase is more susceptible at 30min and 60min. Figure 18 is a plot of inhibition efficiency of 50ppm sulphonic in 1M NaOH against temperature. From the graph there is a magnificent increase in inhibition efficiency as temperature rises at 30min immersion time, at 60min it increased as temperature increased but not as magnificent as that of 30min, but at 90min there is no observable coverage at 120min the inhibition efficiency increased as temperature rise and tends to reduce at 150min. Figure 19 is a plot of inhibition efficiency of 100ppm of sulphonic acid in 1M NaOH against temperature. It can be observed that inhibition efficiency increases as temperature increases at immersion periods of 30min, 60min, 90min, and 120min but tends to decrease at 150min. In figure 20 there is a plot of inhibition efficiency of 150ppm of sulphonic acid in 1M NaOH against temperature. The results from this figure show a tremendously increase in inhibition efficiency at 30min, moderate increase in inhibition efficiency as temperature increase at 60min, very slight increase in inhibition efficiency as temperature rises at 90min and 120, then a decrease in inhibition efficiency temperature increases at 150min of immersion. Figure 21 is a graph of inhibition efficiency of 200ppm of Sulphonic acid in 1M NaOH against temperature. The result show a magnificent increase in inhibition efficiency as temperature rises at 30min a moderate increase at 60min, 90, and 120min and at 150min inhibition efficiency tend to decrease as temperature rises. These observations from these figures (14 to 21) show that sulphonic is chemically adsorbed to the aluminum surface at the early stage of immersion but tend to be physically adsorbed as immersion period lingers.This observation can be explained with respect to the characteristic features of the cathodic process of hydrogen evolution where the decrease or reduction with rise in temperature leads to an increase in the rate of the cathodic reaction. [19]. This effect far over shadows the adsorption and inhibitive effect of sulphonic acid as the time if immersion lingers, because the enhanced rates of hydrogen gas evolution increasingly agitate the interface, which hinders inhibitor adsorption also promotes dispersal of adsorbed inhibitor, [16]. Thus observed tendency of the inhibition efficiency to reduce its rate of increase as temperature rises, tending to reduce with rise in temperature could be attributed to the shift of the adsorption desorption equilibrium towards desorption. This behavior suggests that sulphonic acid was chemically adsorbed on the metal surface at the start of reaction but tend to change to physisorption close to the end of the experiment, [4, 10, 11, 12, 14, 15, 16 & 17].
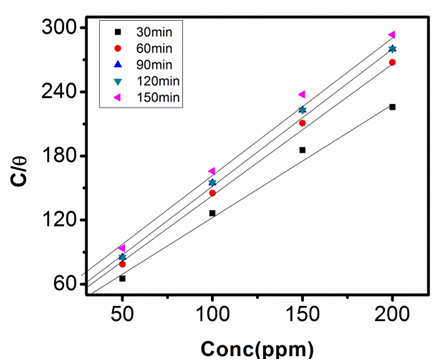 | Figure 13. Langmuir Adsorption Isotherm in 1M NaOH at 313K |
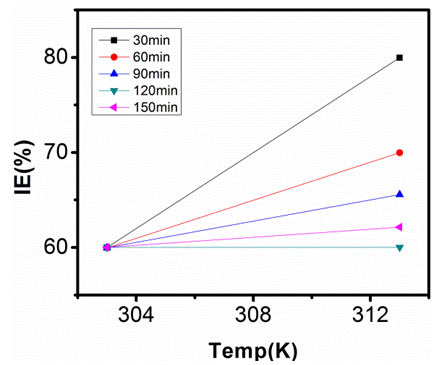 | Figure 14. Inhibition Efficiency of 50ppm Sulphonic Acid against Temp. in 0.5M NaOH |
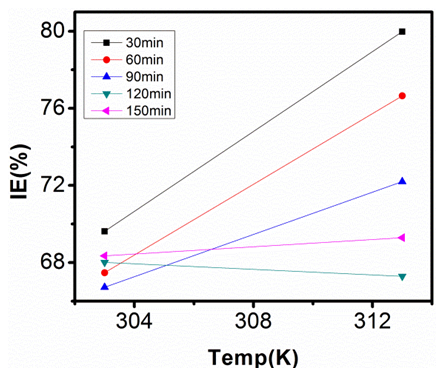 | Figure 15. Inhibition Efficiency of 100ppm Sulphonic Acid against Temp. in 0.5M NaOH |
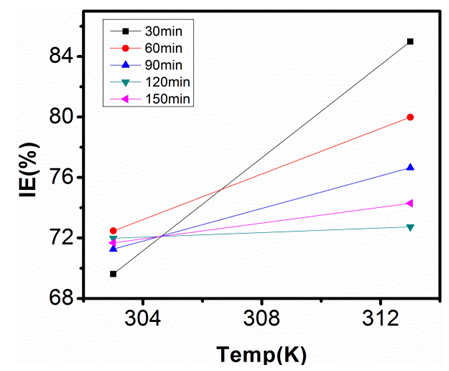 | Figure 16. Inhibition Efficiency of 150ppm Sulphonic Acid against Temp. in 0.5M NaOH |
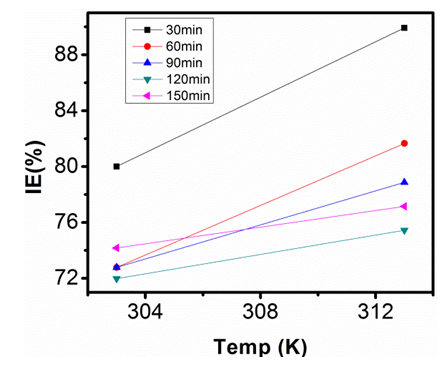 | Figure 17. Inhibition Efficiency of 200ppm Sulphonic Acid against Temp. in 0.5M NaOH |
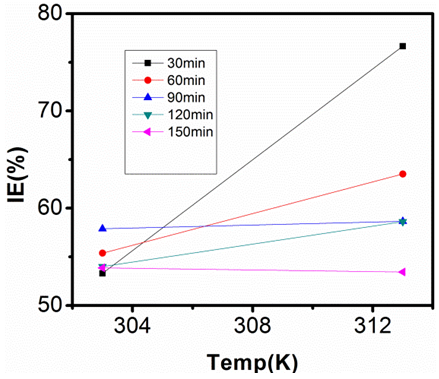 | Figure 18. Inhibition Efficiency of 50ppm Sulphonic Acid against Temp. in 1M NaOH |
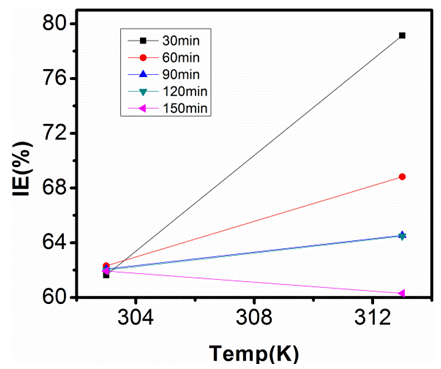 | Figure 19. Inhibition Efficiency of 100ppm Sulphonic Acid against Temp. in 1M NaOH |
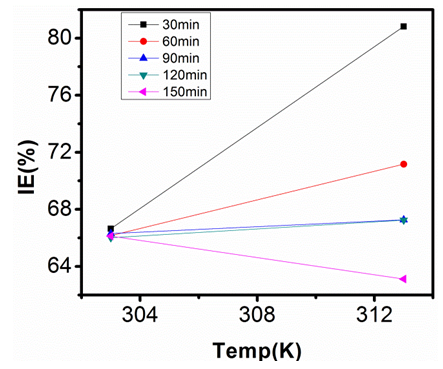 | Figure 20. Inhibition Efficiency of 150ppm Sulphonic Acid against Temp. in 1M NaOH |
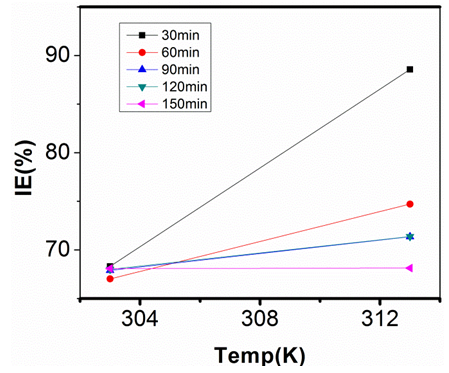 | Figure 21. Inhibition Efficiency of 200ppm Sulphonic Acid against Temp. in 1M NaOH |
3.5. Thermodynamic Parameters
- The relation between corrosion rate and temperature is often expressed by the Arrhenius equation.
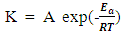 | (7) |
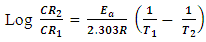 | (8) |
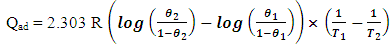 | (9) |
|
|
|
|
4. Conclusions
- Based on the observations made from studies conducted and results obtained, the following conclusions can be drawn.1. Sodium hydroxide corrodes aluminium metal.2. Sulphonic acid inhibits corrosion of aluminium in NaOH and the inhibition efficiency (IE) of sulphonic acid increased with increase in concentration of sulphonic acid.3. Inhibition efficiency increased with rise in temperature below 120min of immersion but tend to decrease between 120min and 150min.4. From the trend of activation energy and heat of adsorption coupled with inhibition efficiency temperature variation the inhibitor molecules were said to be chemically adsorbed to the metal surface below 120min period of immersion but between 120min and 150min mixed adsorption mechanism of physisorption and chemisorptions were involved. 5. The adsorption of the inhibitor molecules fits in Langmuir adsorption isotherm.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML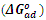 of sulphonic acid on aluminium in 0.5M NaOH solution at 303k
of sulphonic acid on aluminium in 0.5M NaOH solution at 303k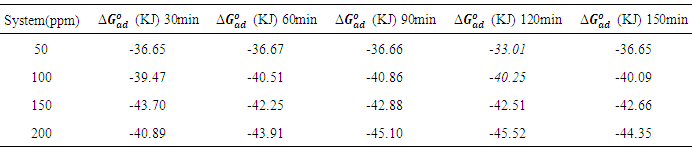
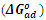 of sulphonic acid on aluminium in 0.5M NaOH solution at 313k
of sulphonic acid on aluminium in 0.5M NaOH solution at 313k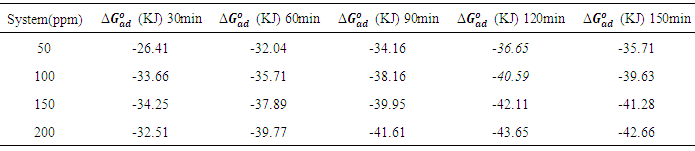
 of sulphonic acid on aluminium in 1M NaOH solution at 303k
of sulphonic acid on aluminium in 1M NaOH solution at 303k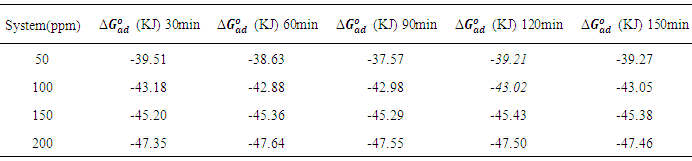
 of sulphonic acid on aluminium in 1M NaOH solution 313K
of sulphonic acid on aluminium in 1M NaOH solution 313K