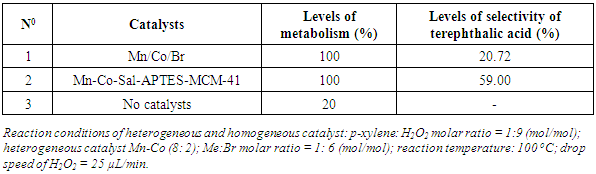-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Physical Chemistry
p-ISSN: 2167-7042 e-ISSN: 2167-7069
2016; 6(1): 1-10
doi:10.5923/j.pc.20160601.01

Influence Parameters on Liquid Phase Reaction Preparing Terephthalic Acid from P- Xylene over Metal Complexes-MCM-41 Catalysts
Nho Dung Nguyen1, Van Thi Tran Thi1, Xuan Nui Pham2, Tam Toan Tran Thanh1
1Department of Chemistry, College of Sciences, Hue University, Hue City, Vietnam
2Department of Oil Refining and Petrochemistry, Hanoi University of Mining and Geology, Hanoi, Vietnam
Correspondence to: Nho Dung Nguyen, Department of Chemistry, College of Sciences, Hue University, Hue City, Vietnam.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The ordered mesoporous silica MCM-41 was anchored with metal-Shiff base complexes to produce metal- Schiff base- MCM-41 catalyst (Mn-Sal-APTES-MCM-41 and Co-Sal-APTES-MCM-41). The manganese or colbalt-Schiff base was synthesized from salicylaldehyde (Sal), (3-aminopropyl)triethoxysilane (APTES) in ethanol and manganese(II) acetate or cobalt (II) acetate. Powder X-ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM), nitrogen adsorption – desorption analyses confirm the retaining of support properties after anchoring. The incorporation of Schiff base complex into the matrix causes a decreasing in the mesoscopic order, the volume of adsorbed nitrogen, the pore volume, the specific surface area, the pore diameter and a increasing in the wall thickness. The DTA-TGA and the FT-IR revealed the incorporation of the manganese or cobalt-Shiff base complex within the pores of MCM-41. On these catalysts, the liquid-phase oxidation reactions of p-xylene to terephthalic acid with hydrogen peroxide over obtained Shiff base complexes showed that distribution of p-xylene oxidation catalyzed by manganese - cobalt complexes (8:2) greatly differs from that by cobalt or manganese complex. Especially, cobalt-manganese complexes catalysts show higher activity and selectivity than neat metal acetate salts for the oxidation reaction of the methyl group under mild condition. At the reaction conditions of heterogeneous catalyst: p-xylene: H2O2: solvent molar ratio = 1:9:5 (mol/mol/mol); heterogeneous catalyst Mn-Co (8: 2); p-xylene: catalyst weight = 1:5 (mol/g); Me: Br molar ratio = 1: 6 (mol/mol); reaction temperature: 100°C; drop speed of H2O2 = 25 µL/min; reaction time: 72 hours.
Keywords: Manganese and cobalt Shiff base-MCM-41 catalyst, p-xylene liquid oxidation
Cite this paper: Nho Dung Nguyen, Van Thi Tran Thi, Xuan Nui Pham, Tam Toan Tran Thanh, Influence Parameters on Liquid Phase Reaction Preparing Terephthalic Acid from P- Xylene over Metal Complexes-MCM-41 Catalysts, Physical Chemistry, Vol. 6 No. 1, 2016, pp. 1-10. doi: 10.5923/j.pc.20160601.01.
Article Outline
1. Introduction
- The selective oxidation of aromatic hydrocarbons into the corresponding oxygen-containing compounds such as alcohols, aldehydes and carboxylic acids is animportant industrialarea and also an academic interest [1]. In particular, the products of selective oxidation reaction of p-xylene are taken a high interest since alcohols, aldehydes for pharmaceutical industry andespecially, terephthalic acid for generous use in the polymer industry. The industrial production of alcohols, aldehydes or terephthalic acid is carried out mainly by oxidation of p-xylene in the presence of metal complexes as homogeneous catalysts [2-4]. These transition metal complexes are good oxidation catalysts. However, these homogeneous catalysts suffer from several problems, such as difficult separation and non-reusable ability. It would be desirable to develop more eco-friendly reusable heterogeneous catalysts for this process because most of homogeneous catalysts have environmental disadvantages. These problems can be solved by anchoring the homogeneous catalysts onto chemically modified support materials. Mesoporous silica materials have attracted attention due to their large tunable pore dimensions, high surface and diversity in surface functionalization [5]. They are also ideal supports for preparing catalysts containing anchored transition metal complexes for some different reactions [6].Several studies have been reported as somesuch “pseudo-heterogeneous” catalystscontaining metal complexes [6-9]. However, there are few reports on anchored metal complexes onto mesoporous materials for the oxidation catalysis of aromatic hydrocarbons.
2. Experimental
2.1. Materials
- All reagents: Cetyltrimethylammonium bromide (CTAB, 99%), tetraethylorthosilicate (TEOS), 3-aminopropyltrietylsilane (APTES), salicylic aldehyde (Sal), manganese acetate Mn(CH3COO)2.4H2O, cobalt acetate Co(CH3COO)2.4H2O, standards of p-xylene and terephthalic acid, solvents such as acetonitrile, ethyl acetate, dimethyl sulfoxide, acetic acid... were purchased from the Merck, Sigma-Aldrichor Fluka Chemical Companies. Reagents were used without extra purification, but solvents were purified by distillation. Solvents and standards (p-xylene and acid terephthalic) were tested by LC-MS.
2.2. Catalyst Preparation
2.2.1. Synthesis of Si-MCM-41
- Si-MCM-41 was synthesized according to the method previously described by Dong Ho Park [5], Parida K.M. et al. [10]. To remove template (CTAB), 1 gram of product after drying was extracted several times with a mixture of ethanol/HCl ratio 100:1 (v: v) (100 mL ethanol and 1 mL 38% HCl) at 80°C for 6 hours.
2.2.2. Synthesis of Metal-Schiff Base Complexes
- The Mn-Sal-APTES and Co-Sal-APTES complexes were preparedusing procedure for metal-Schiff base complexes in literature [11, 12] with a slight modification. The received complexes were defined by their melting points, UV-Vis and IR spectroscopies.
2.2.3. Synthesis of Me-Sal-APTES-MCM-41 (Me as Co or Mn) Catalysts
- Activated MCM-41 (3 g) was suspended in the absolute ethanol solution containing Schiff base complex. The mixture was stirred for 24 hrs. Ethanol was removed using a rotary evaporator and the resulting solid product dried at 80°C in 24 hrs. The final product was washed with ethanol and distilled water until the washings were colourless to ensure that the non-covalently grafted complex and physisorbed metal species were removed. Further drying was carried out in an oven at 80°C for 8 hrs [13].
2.3. Characterizations of Catalysts
2.3.1. Quantification of the Mount of the Metal in Catalyst Samples and Determination of Material Morphology
- The catalyst sample (0.2 g) was digested by HNO3/H2SO4 (3:1, v/v), then the mixture was filtered. Repeat until remaining residue was colourless. The total of the metal was determined by flame atomic absorption spectroscopy of Perkin Elmer 3110 at the wavelength of 240.7 nm.Powder X-ray diffraction patterns were recorded on a D8-Advance Bruker with CuKα radiation (λ = 1.5406 nm). N2 adsorption – desorption isotherms were obtained on a Micromeritics Tristar 3000 sorptometer. Prior to analysis, the sample were outgassed at 180°C in 6 hrs. BET surface areas were calculated from the linear part of the BET plot. Pore size distributions were calculated using the adsorption branches of the N2 isotherms and the Barret-Joyner-Halenda (BJH) method. The images of scanning electron micrograph (SEM) and transmission electron microscopy (TEM) were taken using a Hitachi S4800, operating at an accelerating voltage of 200 kV.
2.3.2. Determination of the Interaction between Grafted Complex and the Surface of Si-MCM41
- Infrared data were registered on KBr pallets using a Shimadzu IR Prestige-21 spectrometer (Japan). Thermal gravimetric and differential thermal analysis (TG-DTA) were performed with a TG Setaraminstrument (France) in the air of argon (100-1000°C).
2.4. Catalytic Oxidation of P-Xylene in Liquid Phase
2.4.1. Catalytic Oxidation Reaction
- Oxidation reaction of p-xylene was carried out according to the following procedure: 20 mL of p-xylene and precisely weighed catalyst were added into a 100 mL tree-neck flask with magnetic stirring. Hydroperoxide was introduced into the flask at a rate while the temperature was kept at a defined value. The contents of hydroperoxide were determined by iodometric method.
2.4.2. Analysis of Oxidation Products
- The oxidation products of p-xylene were identified by GC-MS 7890A-5975C with a (30 m x 250 μm x 0.25 μm) HP-5MS column, 45-250°C (45°C in 2 mins, 4°C /min to 200°C, 15°C /min to 250°C and kept in 5 mins) and by LC-MS co-injection of standard compound. They were quantified by LC-MS Series 2A Shimadzu (Japan) with UV 254 nm) detector and SPD-10ADVP column, eluent consisting of 1% acetic acid: acetonitrile with ratio 20: 80 to 40: 60 (w/w); with flow rate of 1 mL to 1.5 mL. min-1.
3. Results and Discussion
3.1. Synthesis and Characterization of Me-Sal-APTES-MCM-41 (Me as Co or Mn) Catalysts
3.1.1. XRD of Me-Sal-APTES-MCM-41 (Me as Co or Mn) Catalysts
- The small angle XRD patterns of Si-MCM-41, Co-Sal-APTES-MCM41 and Mn-Sal-APTES-MCM-41 samples containing different molar contents of metal-Schiff base complex are shown in Figure 1.
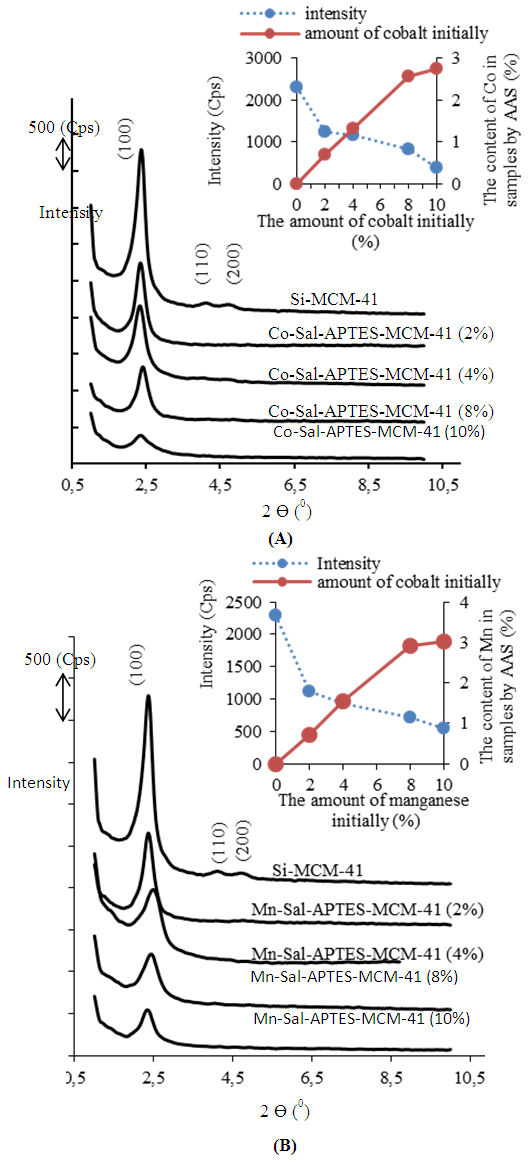 | Figure 1. The X-ray diffraction patterns and the content of metal in samples (determined by AAS) of: (A) Co-Sal-APTES-MCM-41, (B) Mn-Sal-APTES-MCM-41 |
3.1.2. The Nitrogen Adsorption - Desorption Isotherms and Pore Size Distributions
- Nitrogen adsorption-desorption diagrams are given in Figure 2 show all the samples exhibited typical IV shape isotherms of mesoporous framework. The parameters obtained from the schema nitrogen adsorption-desorption are given in Figure 2. The pore diameter of Si-MCM-41 was 23 Å, it is increased with the presence of cobalt complexes (35 Å) and manganese complexes (39.4 Å) in the pores. It is in accordance with the classification of IUPAC mesoporous materials [15], the hexagonal pores remained intact. However, the foot of the pore size distribution curves of modification materials was larger, demonstrating that the regular arrangement of the pores was lower. The pure silica material had a sharp capillary condensation steps at relative pressures of 0.2 < p/p0< 0.4 and a large surface area (971.60 m2/g). The shift to low relative pressure of p/p0 > 0.4 was demonstrated the introduction of bulky metal organic complex. The other hand, the surface area of modified samples obtained from the schemes was 375.96 m2/g and 581.60 m2/g, it strongly decreased compared to that of Si-MCM-41. These data were consistent with the XRD results and assigned to the presence of metal organic complexes which may have been modified into the capillary of materials.
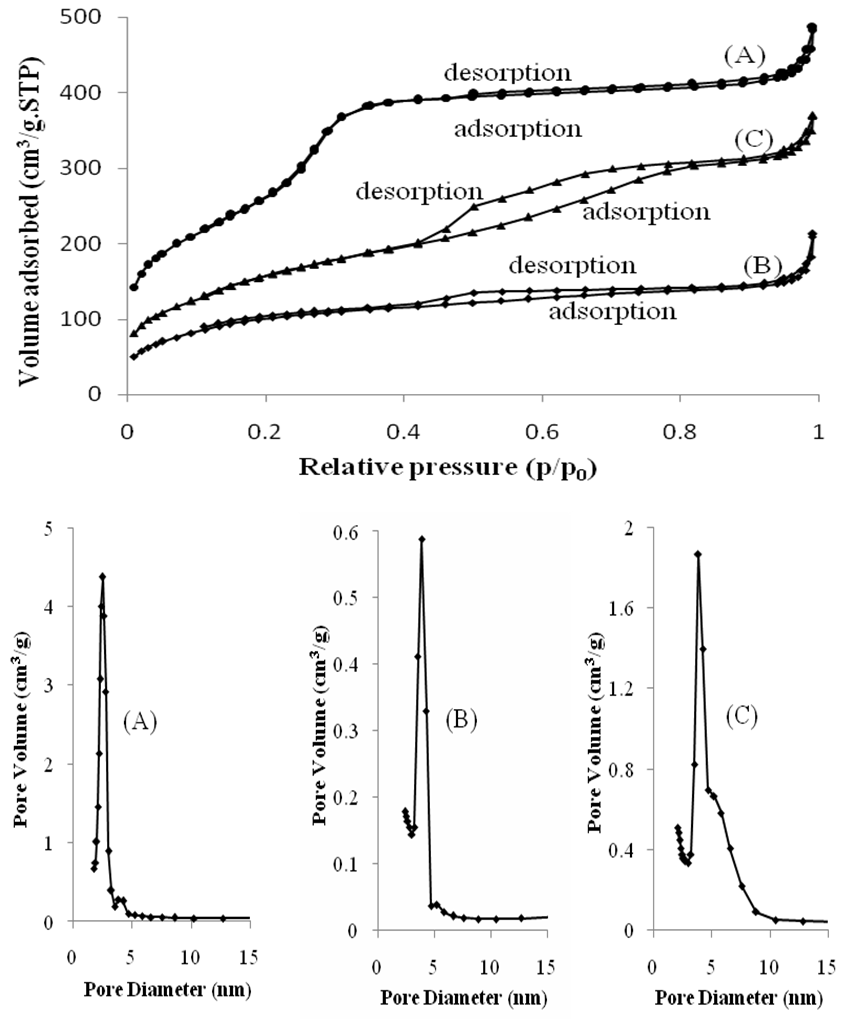 | Figure 2. The nitrogen adsorption – desorption isotherms and pore size distributions of: (A) Si-MCM-41, (B) Co-Sal-APTES-MCM-41, (C) Mn-Sal-APTES-MCM-41 |
3.1.3. The SEM and TEM Images
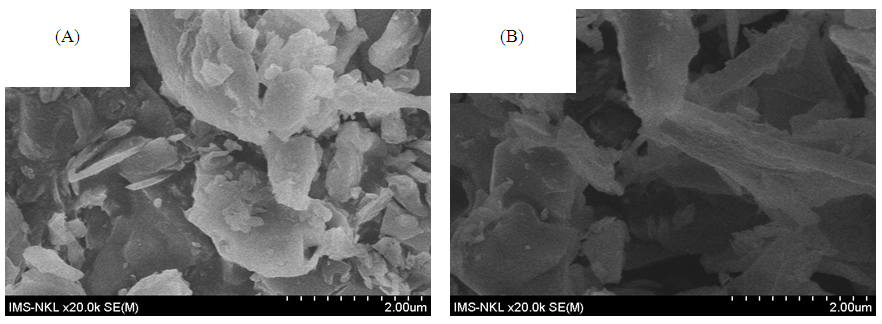 | Figure 3. The SEM images of (A): Si-MCM-41; (B): Mn-Sal-APTES-MCM-41 |
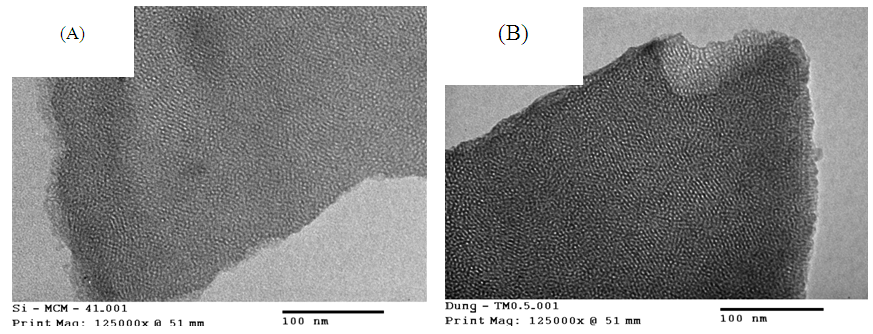 | Figure 4. The TEM images of (A): Si-MCM-41; (B): Mn-Sal-APTES-MCM-41 |
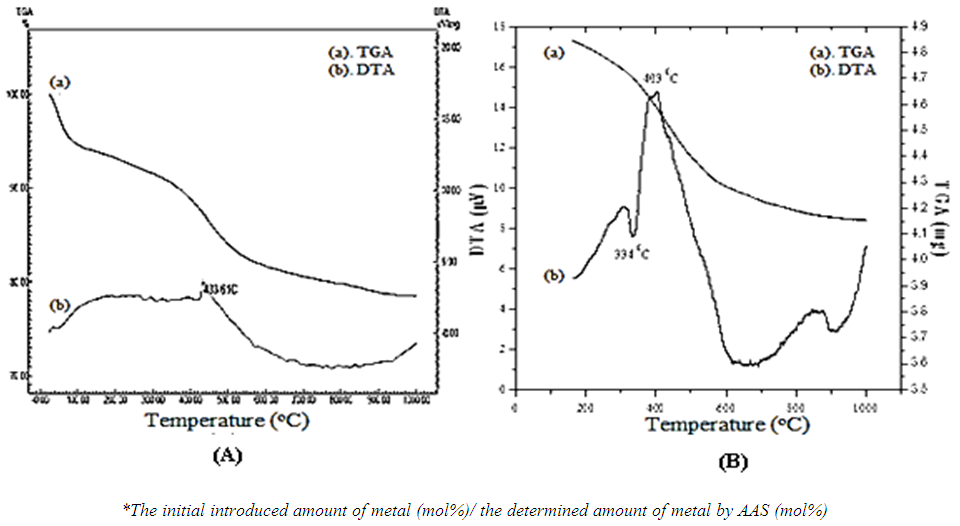 | Figure 5. The TGA-DTA diffractions of: (A) Co-Sal-APTES-MCM-41 (0.2/0.70)*; (B) Mn-Sal-APTES-MCM-41 (0.2/0.73)* |
3.1.4. The TGA-DTA Diffractions
- The TGA-DTA curves of some catalysts are shown in Figure 5. Both the samples were seen a mass loss below 100°C due tothe remove of pthysically adsorped water and solvent inside the pores. In the TGA-DTA of Co-Sal-APTES-MCM41, there was a large mass loss at 433.6°C and in the Mn-TGA-DTA, that was at 334- 403°C, those due to the decomposition of the Sal-APTES ligands. These values were much higher than the boiling temperature of pure Sal and pure APTES (197 and 215°C at 760 mmHg, respectively). These may be the signs indicating that there were formed co-valent chemical bonds between the metal organic complex and the silica surface of MCM-41. The total of the mass loss of organic ligand for cobalt catalyst was 21.504%. The UV-Vis-DRS showed that there were Co(II) and Co(III) state, so that the content of cobalt in the Co-Sal-APTES-MCM-41 was about 0.126 mmol to 0.284 mmol, this value to suit the result determined by AAS was 0.176 mmol.g-1 (0.70 mol%). Similary, the total of the mass loss for manganese catalyst was 12.169%, so that the content of manganese in the Mn-Sal-APTES-MCM-41 was about 0.182 mmol.g-1, that is equal to the result determined by AAS (0.73 mol%).
3.1.5. The FT-IR Spectra
- The FT-IR spectra of Si-MCM-41 support, Me-Sal-APTES complexes and Me-Sal-APTES-MCM-41 catalysts are performed in the Figure 6. It could be seen that the bands can be attributed to the vibrations of O-H silanol groups, Si-O-Si inthe inorganic support and in the organic complex. Besides, it is constantly concerned with the weak absorption bandat 2970-2935 and 1540-1440 cm-1 corresponded to the stretching and bending C-H vibrations. The sharp peak at 1653-1627 cm-1 due to a C=N stretching vibration and the absorption peaks of the Me-O and Me-N were present in all spectra of metal complexes and catalysts. These confirmed the attachment of the metal organic complexes onto the surface of MCM-41.
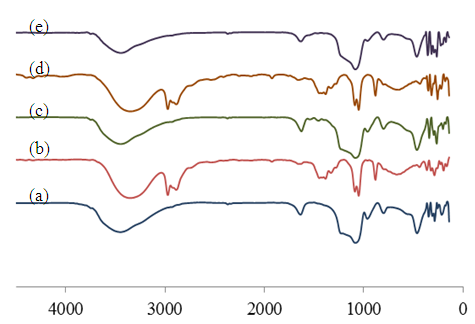 | Figure 6. The FT-IR spectra of: (a)Si-MCM-41, (b) Co-Sal-APTES, (c) Co-Sal-APTES-MCM-41 catalyst, (d) Mn-Sal-APTES, (e) Mn-Sal- APTES-MCM-41 catalyst. |
3.2. Oxidation Reaction of p-Xylene to Terephthalic acid over Me-Sal-APTES-MCM-41 Catalysts
3.2.1. Effect of Solvents
- Sovent plays a important role in reaction of organic syntheisis, the solvent has to possess suitable polarity for p-xylene as well as other polar products transformation. Three solvents provided the convestion of p-xylene to 100% and terephthalic acid was also obtained. The solvent of acetic acid provided the highest selectivity to terephthalic acid (15,73%) in compared with the rest solvent (< 10%). For this reason, acetic acid solvent was chosen for next study (Figure 7).
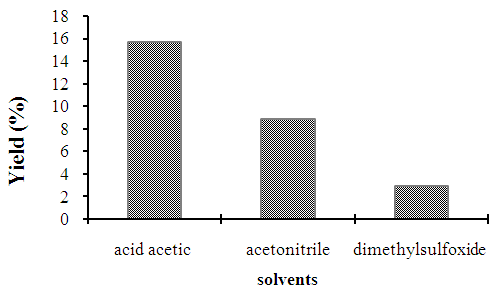 | Figure 7. Effect of solvents on the production yield of terephtalic acid |
3.2.2. Effect of Reaction Temperature
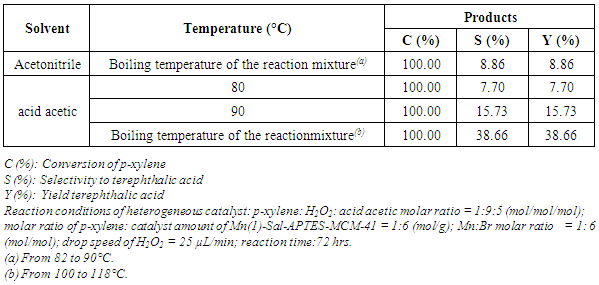
- In the solvent of acetic acid and acetonitril, the yield and selectivity to terephthalic acid increase as the temperature increases. The conversion of p-xylene is appoximately to 100% for all of temperatures studied and terephthalic acid starts to appear. The terephthalic acid yield reaches the highest value of 38.66% at boiling temperature of mixture (Table 1) (acetic acid, p-xylene, H2O2, H2O).
|
3.2.3. Effect of the Oxidant and the Oxidant Molar Ratio
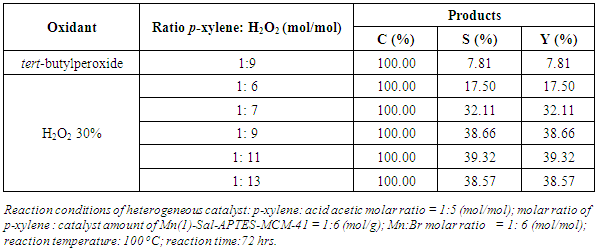
- As the molar ratio of p-xylene: H2O2 vary from 1:6 to 1:13 the conversion reaches 100% and terephthalic acid is obtained. Table 2 shows that the terephthalic acid yield increases significantly as the amount of H2O2 increases and the yield increases in significantly as the amount of H2O2 excesses the molar ratio of 1:9. Tert-butylperoxide as a oxidant for ethylbenzene, cyclohexene, benzyl alcohol using nano -Co(II) was reported by [16]. In the present paper, the conversion of p-xylene reaches 100% in both of H2O2 and tert-buthylperoxide as oxidant but H2O2 provides better selectivity to terephthalic acid. Then, H2O2 as oxidant with drop rate of 25 (μL/min) and molar ratio of p-xylene: H2O2 of 1:9 were chosen for next study.
|
3.2.4. Effect of Accelerators and Ratio of Accelerators
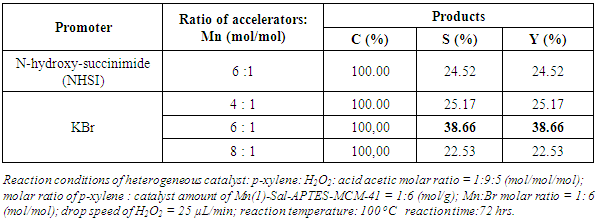
- The molar ratio of Br:Mn = 6:1 provided the best oxidation in studied range. Then, we conducted the reaction under the condition with the molar ratio of Br:Mn = 4:1; 6:1; 8:1 (Table 3). Terephthalic acid was obtained in all cases in which the catalyst with the molar ratio of 6:1 exhibited best yield in compared that with the molar ratio of 8:1 indicating that the increase in Br leads to the reduce in yield. It could be explained that the high amount of Br supply the high amount of Bro radicals produce, as results, the Br radicals reacted together forming Br2.
|
3.2.5. Effect of Content of Manganese in Catalysts
- The conversion of p-xylene over all catalyst samples at reaction condition is 100%. The selectivity of terephthalic acid reaches 38.66% on 2% manganese in Mn-Sal-APTES-MCM-41. The diffusion of p-xylene and products are under the strong influence of the present of Sal-APTES complex moleculars grafted on the surface and into MCM-41 mesopores, so that the content of manganese in catalyst samples is higher, the productivity of terephthalic acid is lower (Figure 8).
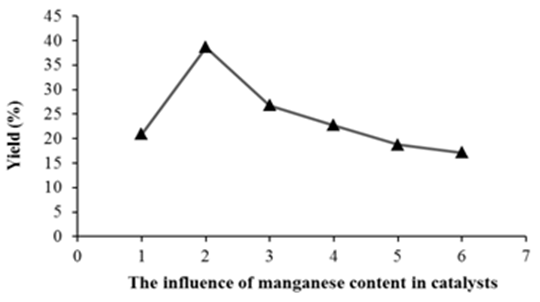 | Figure 8. The influence of manganese content in catalysts on the yield of terephthalic acid |
3.2.6. Effect of the Replace Manganese by Cobalt in Catalysts
- The replace of manganese in catalysts by corelative cobalt, the results of reaction is showed on Figure 9.
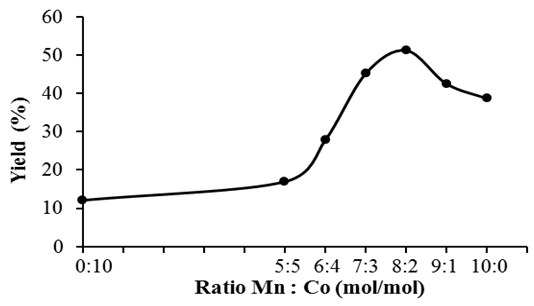 | Figure 9. Effect of ratio Mn: Co in catalysts on the yield of terephthalic acid |
3.2.7. Effect of the Catalyst Weight, Solvent and Reaction Time
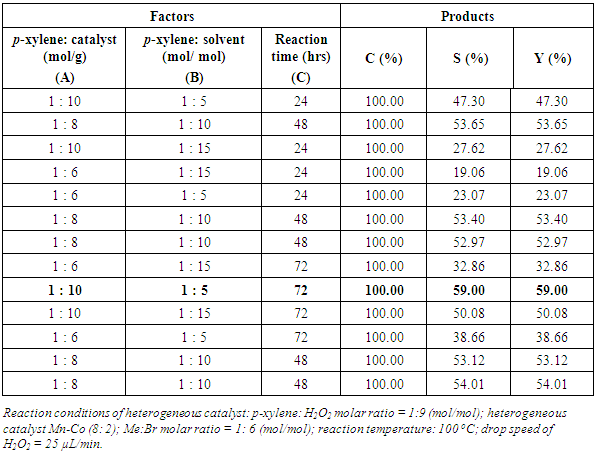
- The effect of factors into terephthalic acid yield was estimated by optimal experiments with orthogonal of three factors with 5 points at center (Table 4). The regression equation was obtained by Minitab-15 as follows:H = 37.206 + 8.794A – 4.801B + 7.944C – 2.349AB + 0.596AC + 1.121BC + 1.569ABCWith significant level of 0.05 p-value of coeficients is less than 0.05 indicating that all of factors contribute significantly in yield. The yield of terephthalic acid reaches 59% in experimental reaction condition.
|
3.3. Survey on Heterogeneity, the Possibility of Recovering and Reusing Catalysts
- When using catalysts containing Schiff bases fixed on the surface of MCM-41, if the link between the Schiff base and the MCM-41 is unstable, during the oxidization reaction, catalytic centers are easily accessible to liquid phase and play as a homogenous catalyst. So, checking reliability, the possibility of recovering and reusing catalysts is an important issue in the process of studying reactions in the presence of solid catalysts.The oxidization reaction of p-xylene with a catalyst Mn-Co-Sal-APTES-MCM-41 is surveyed in the following reaction conditions:- p-xylene ratio: H2O2: acetic acid solvent = 1: 9: 5 (mol/mol/mol)- P-xylene ratio: Mn-Co catalyst molar (1) = 1: 6 (mol/g)- Reaction temperature: 100°C - Reaction time: 24 hours- Speed of H2O2 into the reaction system: 25 µL/ / min
3.3.1. Survey Results on Heterogeneity of Catalyst Mn-Co-Sal-APTES-MCM-41
- After some time of conducting the reaction, a mixture of reactions is centrifuged, filtered to separate the catalyst. A mixture of reactions (no catalysts) continues to be conducted, stirred and heated at 100°C for 24 hours, samples are taken at various time intervals for analysis by HPLC. The results show that the level of metabolism of p-xylene does not change after 24 hours of reaction, so the Schiff base is not dissolved out of heterogeneous catalyst during the reaction. This is again proved by determining the content of metal in the solution after the reaction. By means of analyzing atomic absorption spectroscopy (AAS) on samples of the solution after the reaction, the results show that the content of metal in the solution after the reaction is undetected by this method (Figure 10).
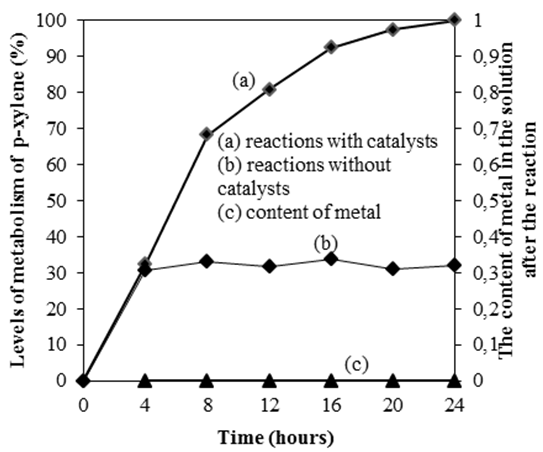 | Figure 10. Checking of heterogeneous catalysiscatalyst (Mn-Co-Sal- APTES-MCM-41) |
3.3.2. Survey Results on the Possibility of Recovering and Reusing Catalysts
- Recovering and reusing metal catalysts are of great importance in limiting waste which seriously affects the environment and in saving costs. Besides, the recovery of catalysts limits the phenomenon of products being infected by metals. Due to the high possibility of reusing, catalysts on heterogeneous substances are always prioritized to use in processes of conducting organic synthesis reactions.The possibility of recovering and reusing catalysts for the oxidization of p-xylene is surveyed. After finishing the initial reaction, a mixture of reactions is centrifuged and catalysts are recovered for the next surveys. After the recovery, catalysts are washed many times by distilled water, acetone, n-hecxan to remove solvents and reactants, dried overnight at 100°C and reused in the new reactions in the same initial conditions. In this study, the author reuses catalysts three times; in each survey, catalysts are characterized by XRD to demonstrate the structure of the material after conducting the reaction, the results are shown in Figure 11 and Figure 12.
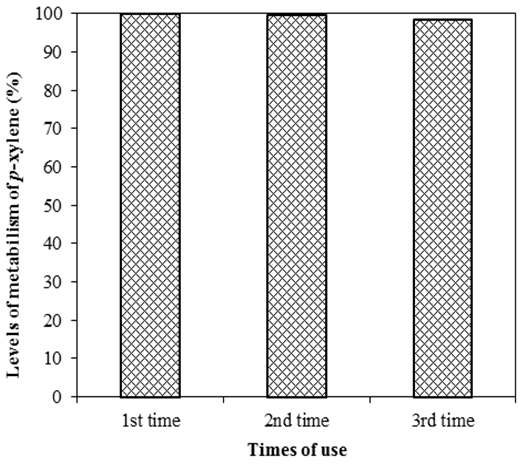 | Figure 11. Survey on the possibility of reusing catalyst Mn-Co-Sal- APTES-MCM-41 |
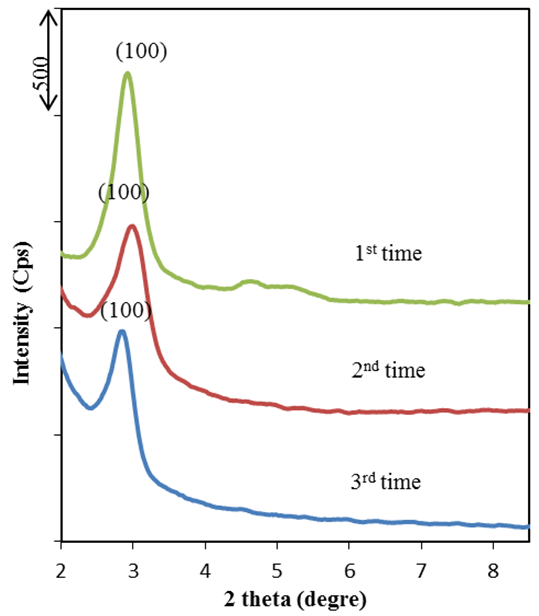 | Figure 12. Schema of XRD after three times of reusing catalyst Mn-Co-Sal-APTES-MCM-41 |
3.3.3. Research Results on Comparing Performance of Creating Terephthalic Acid on Systems of Heterogeneous and Homogeneous Catalysts in the Same Reaction Conditions
- To compare the effectiveness of the oxidization reaction of p-xylene which creates terephthalic acid on systems of heterogeneous and homogeneous catalysts in the same reaction conditions, the results are shown in Table 5.
|
4. Conclusions
- The ordered mesoporous silica MCM-41 was anchored with manganese or cobalt-Schiff base complexes to produce metal- Schiff base- MCM-41 catalyst (Mn-Sal-APTES-MCM-41 and Co-Sal-APTES-MCM-41). The manganese or colbalt-Schiff base was synthesized from salicylaldehyde (Sal), (3-aminopropyl)triethoxysilane (APTES) in ethanol and mangan acetate or cobalt acetate. Powder X-ray diffraction, scanning electron microscopy (SEM) and transmission electron microscopy (TEM), nitrogen adsorption – desorption analyses confirm the retaining of support properties after anchoring. The incorporation of Schiff base complex matrix causes a decreasing in the mesoscopic order, volume of adsorbed nitrogen, the pore volume, the specific surface area, the pore diameter and a increasing in the wall thickness. The DTA-TGA and the FT-IR revealed the incorporation of the manganese or cobalt-Schiff base complex within the pores of MCM-41. On these catalysts, the liquid-phase oxidation reactions of p-xylene to terephthalic acid with hydrogen peroxide over obtained Shiff base complexes showed that distribution of p-xylene oxidation catalyzed by manganese - cobalt complexes (8:2) greatly differs from that by cobalt or manganese complex.Especially, cobalt-manganese complexes catalysts show higher activity and selectivity than neat metal acetate salts for the oxidation reaction of the methyl group under mild condition. At the reaction conditions of heterogeneous catalyst: p-xylene: H2O2: solvent molar ratio = 1:9:5 (mol/mol/mol); heterogeneous catalyst Mn-Co (8: 2); p-xylene: catalyst weight = 1:5 (mol/g); Me:Br molar ratio = 1: 6 (mol/mol); reaction temperature: 100°C; drop speed of H2O2 = 25 µL/min; reaction time: 72 hours.
ACKNOWLEDGEMENTS
- This work was supported by Department of Chemistry, College of Sciences, Hue University.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML