-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Physical Chemistry
p-ISSN: 2167-7042 e-ISSN: 2167-7069
2015; 5(3): 49-62
doi:10.5923/j.pc.20150503.01

Inhibitive Action of Alhagi Maurorum Plant Extract on the Corrosion of Copper in 0.5 M H2SO4
B. A. Abd-El-Nabey1, S. El-Housseiny1, G. A. El-Naggar2, E. A. Matter2, G. Esmail2
1Alexandria University, Faculty of Science, Chemistry Department, Ibrahimia, Alexandria, Egypt
2Damanhur University, Faculty of Science, Chemistry Department, Damanhur, Egypt
Correspondence to: B. A. Abd-El-Nabey, Alexandria University, Faculty of Science, Chemistry Department, Ibrahimia, Alexandria, Egypt.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The effect of Alhagi Maurorum plant extract on the corrosion of copper in aqueous 0.5M sulphuric acid was investigated by electrochemical impedance spectroscopy (EIS), potentiodynamic polarization, and weight loss techniques. The plant extract act as cathodic-type inhibitor and serve as an effective inhibitor for the corrosion of copper in sulphuric acid media. Theoretical fitting of different isotherms, Langmuir, Flory–Huggins, and the kinetic–thermodynamic model, were tested to clarify the nature of adsorption. The thermodynamic parameters of the corrosion reaction of copper in acidic medium in the absence and the presence of the extract have been determined.
Keywords: Potentiodynamic Polarization, EIS, Weight Loss, Inhibitor, Copper, H2SO4
Cite this paper: B. A. Abd-El-Nabey, S. El-Housseiny, G. A. El-Naggar, E. A. Matter, G. Esmail, Inhibitive Action of Alhagi Maurorum Plant Extract on the Corrosion of Copper in 0.5 M H2SO4, Physical Chemistry, Vol. 5 No. 3, 2015, pp. 49-62. doi: 10.5923/j.pc.20150503.01.
Article Outline
1. Introduction
- Copper is metal that has a wide range of applications due to its good thermic conductivity and mechanical properties. It is used in electronics, for production of wires, sheets, tubes, and also to form alloys. Copper is resistant toward the influence of atmosphere and many chemicals, however, it is known that in aggressive media it is susceptible to corrosion. The use of copper corrosion inhibitors in such conditions is necessary since no protective passive layer can be expected [1]. Inhibitors are widely used in the corrosion protection of metals in several environments. Most of the efficient inhibitors are organic compounds which contain nitrogen, sulphur and/or oxygen atoms in their molecules [2, 3].Nowadays, the uses of some chemical inhibitors have been limited due to their synthesis is often very expensive and they can be toxic or hazardous for human beings and environment as well. Recently, plant extracts, containing mixture of compounds having oxygen, sulphur and nitrogen elements, are employed as corrosion inhibitors in order to develop cleaning chemicals for green environment. Several studies have been published on the use of plant extracts as corrosion inhibitors in different media [4–10]. The possibility of the copper corrosion prevention in different aqueous solutions has attracted many researchers so until now numerous possible inhibitors have been investigated. [11]. These studies reported that there are a number of organic and inorganic compounds which can do that for the corrosion of copper [12-16]. A previous study [17] used electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization techniques to evaluate the inhibitive efficiency of thioxoprymidinone derivatives on the corrosion of copper in sulphuric acid media. The results obtained show that the thioxoprymidinone derivatives could serve as an effective inhibitor for the corrosion of copper in sulphuric acid media.In a recent work obtained from our laboratory [11] we studied the effect of extract of cannabis plant on the corrosion of copper in aqueous 0.5M sulphuric acid using electrochemical impedance spectroscopy (EIS), potentiodynamic polarization, weight loss and optical micrograph techniques. The corrosion rates of copper and the inhibition efficiencies of the extract were determined. The results obtained show that the extract solution of the plant could serve as an effective inhibitor for the corrosion of copper in sulphuric acid media. The inhibition mechanism was discussed.The main objectives of this study were to evaluate the inhibition efficiency of Allhagi Maurorum extract in preventing the acid dissolution of copper metal at different temperatures and to investigate the influence of exposure time on the performance of Allhagi Maurorum extract. Also, the effect of the extract on the mechanism of the corrosion of copper will be taken in consideration.
2. Experimental
2.1. Electrochemical Measurements
- Electrochemical impedance and polarization measurements were achieved using frequency response analyzer (FRA) / potentiostat supplied from Parstate Instrument. The frequency range for EIS measurements was 0.1 × 104 Hz with applied potential signal amplitude of 10 mV around the rest potential. The data were obtained in a three- electrode mode cell; graphite rod and saturated calomel electrodes (SCE) were used as counter and reference electrode. The material used for constructing the working electrode was copper that had the following chemical composition (% wt) 0.5% Ca, 99.5% Cu was used for the electrochemical corrosion studies in aqueous solutions. The working electrode was fabricated by cutting and shaping them in cylindrical forms. A long screw fastened to one end of the test cylinder for electrical connection. The Teflon gasket thereby forms a water-tight seal with the specimen electrode that prevents ingress of any electrolyte and thus avoiding crevice effect. The leak-proof assembly exposes only glass, only one side of rod was left uncovered as constant surface area in contact with the solution. The sample was wet hand-polished using different grade emery papers 320, 400, 600, and 800 grit finishes starting with a coarse one and proceeding in steps to the fine grit up to a mirror finish, washed thoroughly with double-distilled water and finally dried by absolute ethanol, just before immersion in the solution. Each experiment was carried out with newly polished electrode.Before polarization and EIS measurements, the working electrode was introduced into the test solution and left for 20 min to attain the open circuit potential (OCP) at which the change of OCP with time is 2 mV/min, i.e, the system had been stabilized.The polarization curve measurements were obtained at scan rate of 20mV/min starting from cathodic potential (Ecorr -300 mV) going to anodic direction. All the measurements were done at 30.0 ± 0.1°C in solutions open to the atmosphere under unstirred conditions.To test the reliability and reproducibility of the measurements, duplicate experiments were performed in each case of the same conditions.
2.2. Weight Loss Measurements
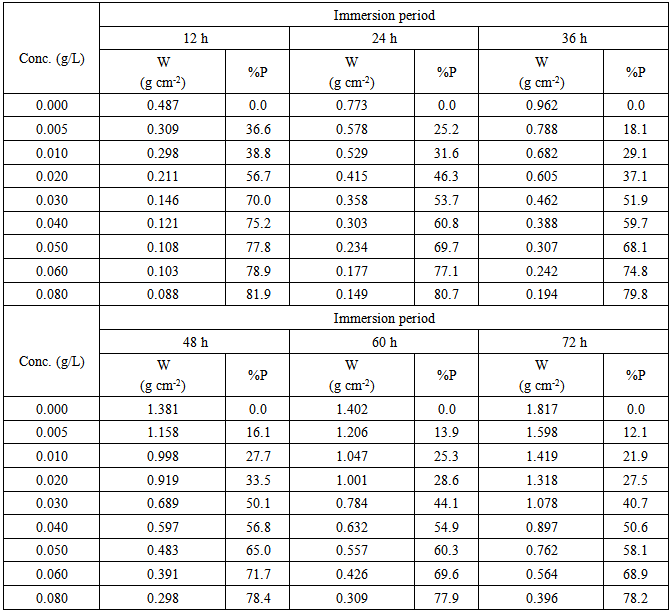
- Coupons with area 2 cm2 rectangular copper and with the same chemical composition of copper samples used in the electrochemical measurements were used in the experiment. The weight loss coupons were polished, cleaned and suspended in beakers containing 100 ml of the test solutions. After definite time, the coupons were removed from the solution, washed with distilled water, ethanol and then dried by acetone and re weighted. The weight loss was then determined (gm/cm2) the experiment was then repeated for different time in travels up to 72 hours. To test the reliability and responsibility of the measurements, duplicate experiments were performed in each case of the same conditions.
2.3. Solution Preparation
- The test solutions were prepared from analytical grade reagents and distilled water: 98% H2SO4 was purchased from Aldrich chemicals. The plant extract was obtained by drying the plant for 1 h in an oven at 80°C and grinding to powdery form. A 10 g sample of the powder was refluxed in 100 mL double distilled water for 1 h. The refluxed solution was filtered to remove any contamination. The concentration of the plant extract was determined by evaporating 10 mL of the filtrate and weighing the residue. Prior each experiment, 5M H2SO4 is added to an appropriate volume of the extract solution and double distilled water to obtain a solution of 0.5M H2SO4 and the required concentration of the extract.
3. Results and Discussions
3.1. Potentiodynamic Polarization Results
- Figure 1 shows the potentiodynamic polarization curves of copper in 0.5M sulphuric acid, in absence and presence of different concentrations of Alhagi Maurorum extract. As seen from the figure, addition of the Alhagi Maurorum extract affects the cathodic part of the polarization curve for copper rather than the anodic one indicating that the Alhagi Maurorum extract could be classified as cathodic-type inhibitor and retard the reduction of oxygen gas at the cathodic areas. The cathodic polarization curves of copper in absence and in presence of the extract show a limiting current, indicating that the cathodic reduction of oxygen gas at the copper surface takes place under diffusion control.
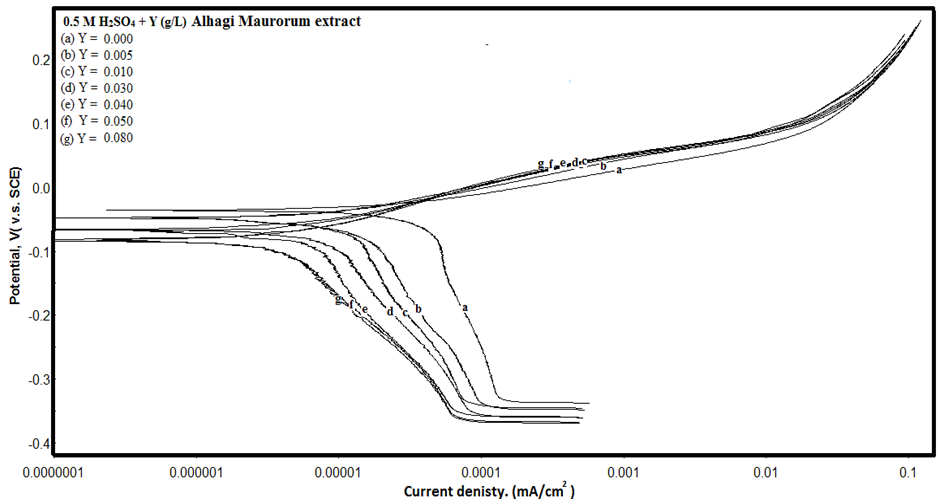 | Figure 1. Potentiodynamic polarization curves of copper in 0.5M sulphuric acid, in absence and presence of different Alhagi Maurorum extract concentrations |
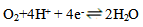 | (1) |
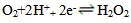 | (2) |
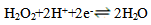 | (3) |
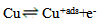 | (4) |
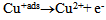 | (5) |
 | Figure 2. Chemical constituents of Alhagi Maurorum extract |
3.2. Electrochemical Impedance Spectroscopy (EIS) Results
- Figure 3 shows the Nyquist impedance plots of copper in 0.5M sulphuric acid, in absence and presence of different concentrations of Alhagi Maurorum extract. The Nyquist plots of copper in acidic medium consist of distorted semicircles followed by diffusion tail indicative that the corrosion process occurs under diffusion control which confirm the results obtained from the polarization measurements. The increase in the size of the semicircle in presence of the extract indicates that a barrier gradually forms on the copper surface.
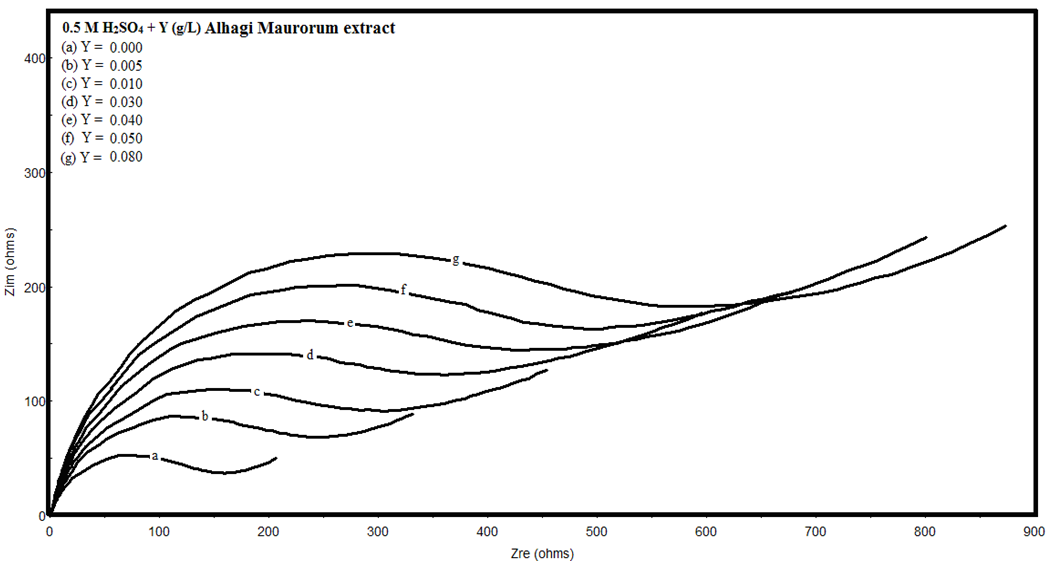 | Figure 3. Nyquist plots of copper in 0.5M sulphuric acid, in absence and presence of different concentrations of Alhagi Maurorum extract |
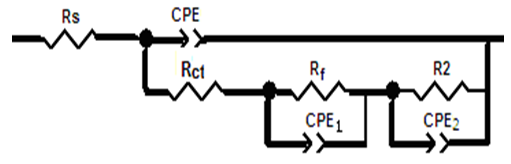 | Figure 4. Schematic for the equivalent circuit of copper |
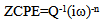 | (6) |
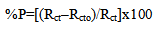 | (7) |
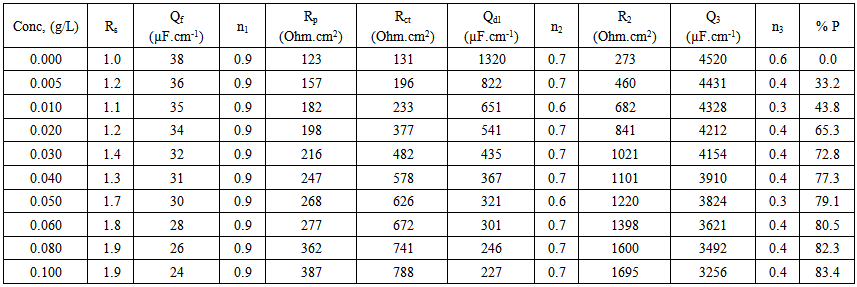 | Table 1. Electrochemical impedance parameters of copper in 0.5M sulphuric acid containing different Alhagi Maurorum extract concentrations |
3.3. Weight Loss Results
- Table 2 shows the variation of weight loss of copper in 0.5M sulphuric acid, in absence and presence of Alhagi Maurorum extract with exposure time up to 72 hours at 30°C. As seen, the weight loss increased with exposure time and decreased by the addition of the extract. The percentage inhibition efficiency (% p) was calculated from the following equation:
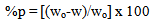 | (8) |
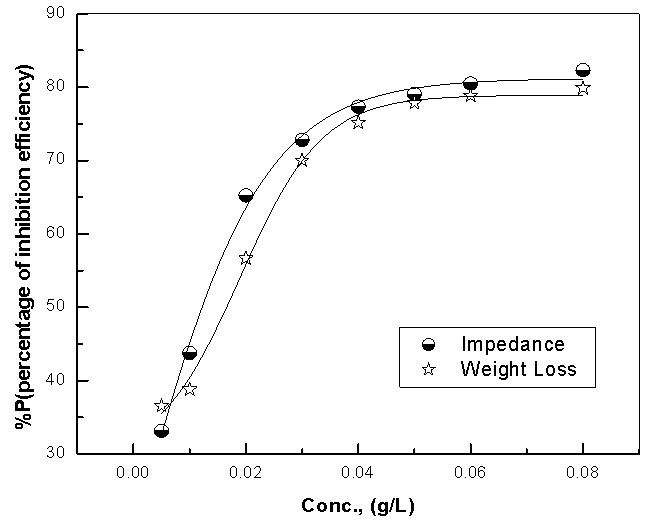 | Figure 5. Relation between the percentage inhibition efficiency and concentration of Alhagi Maurorum extract for copper in 0.5M sulphuric acid solution |
|
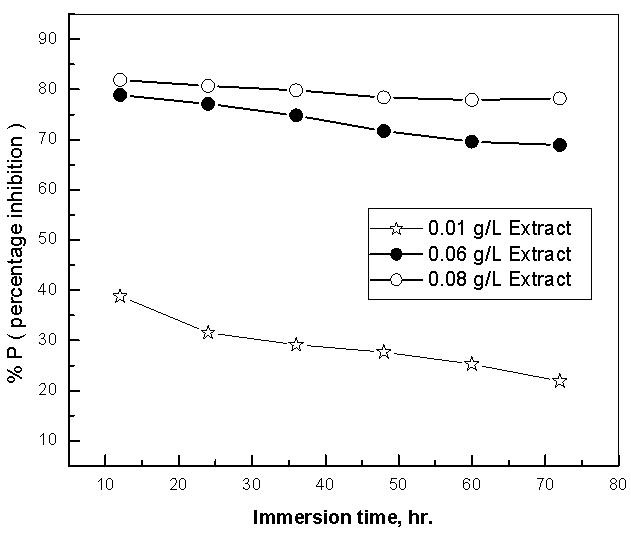 | Figure 6. Dependence of the %P of Alhagi Maurorum extract for copper in 0.5M sulphuric acid on the immersion time |
3.4. Adsorption of Alhagi Maurorum Extract At Copper/Solution Interface
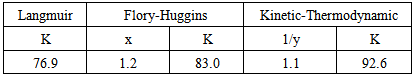
- Application of adsorption isotherms:The understanding of the nature of the adsorption process of various kinds of extracts on metal surfaces was essential to our knowledge of their inhibition action on corrosion. The action of an inhibitor in the presence of aggressive acid media is assumed to be due to its adsorption [24] at the metal/solution interface.The inhibition action was regarded as simple substitutional process, in which an inhibitor molecule in the aqueous phase substitutes an x number of water molecules adsorbed on the metal surface, viz.
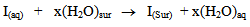 | (9) |
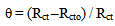 | (10) |
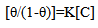 | (11) |
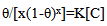 | (12) |
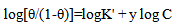 | (13) |
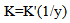 | (14) |
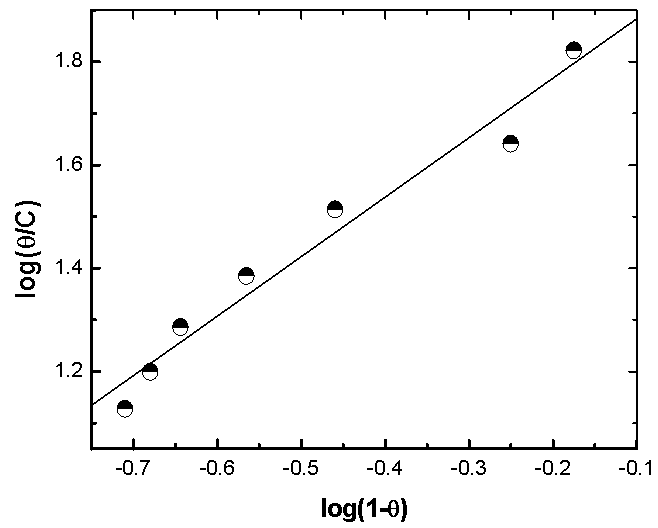
 | Figure 7. Linear fitting of the data of Alhagi Maurorum extract to Langmuir isotherm for copper |
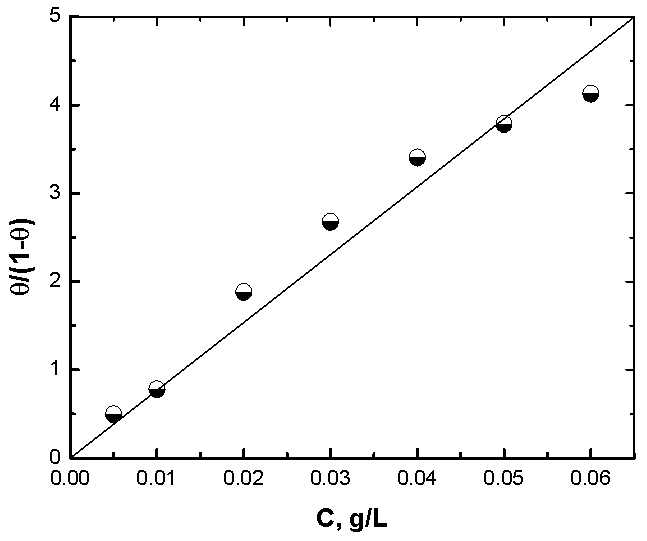
 | Figure 8. Linear fitting of the data of Alhagi Maurorum extract to Flory Huggins isotherm for copper |
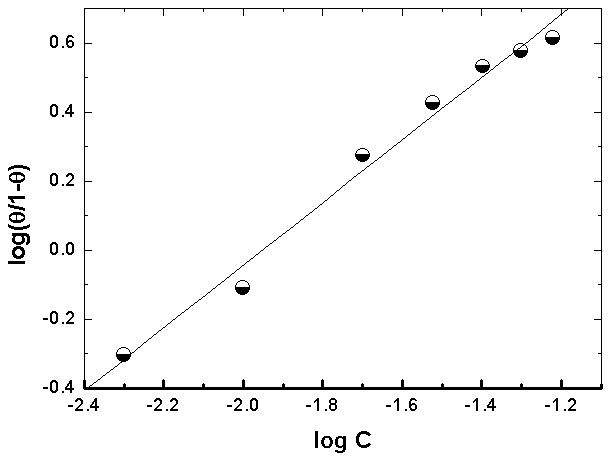 | Figure 9. Linear fitting of the data of Alhagi Maurorum extract to Kinetic-Thermodynamic model for copper |
|
3.5. Effect of Temperature on the Corrosion Behaviouer of Copper in 0.5M Sulphuric Acid in Absence and Presence of Alhagi Maurorum Extract
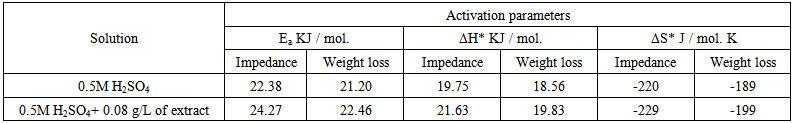
- Many industrial processes take place at high temperatures so, it is particularly important to study the variation of the inhibition efficiency with temperature. When temperature is raised, corrosive action is usually accelerated, particularly in media where evolution of hydrogen accompanies corrosion. Raising the temperature will decrease the inhibitor adsorption on the metal surface; consequently, it will lose its protective action. Alhagi Maurorum plant was used in this investigation as an example of plant extracts, as a corrosion inhibitor.Figures 10, 11 show the Nyquist plots of copper in 0.5M sulphuric acid solution, in absence and in presence of Alhagi Maurorum extract at different temperatures. As seen, the size of the capacitive semicircle decreases with rising the temperature of corrosive medium this may be attributed to the acceleration of the corrosive action of the acid. These plots were analyzed by the equivalent circuit that is previously used, Figure 4. The values of Rct and Cdl of copper in 0.5M sulphuric acid, in absence and presence of Alhagi Maurorum extract at different temperatures are also given in table 4. It is evident that, at given temperature, the charge transfer resistance is higher in presence of Alhagi Maurorum extract than its absence due to the inhibitive effect of the extract. General trend for increasing Cdl values with rising the temperature are also observed as shown in figure 12, this behaviour is probably attributed to the desorption of the adsorbed ions and the extract ingredients from the copper surface with rising the temperature. Table 4 shows also that the efficiency of Alhagi Maurorum extract for the acidic corrosion of copper is slightly increased from 82 to 88% at temperature 30 to 60°C. This behaviour indicates that Alhagi Maurorum extract act as good corrosion inhibitor for copper in 0.5M H2SO4 and its efficiency is nearly constant and independent on the temperature which means that Alhagi Maurorum act as a good inhibitors for the acidic corrosion of copper.
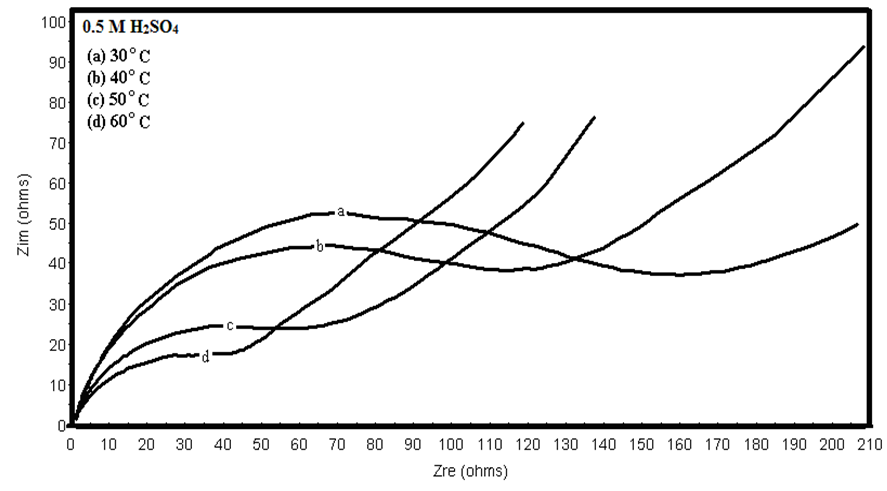 | Figure 10. Nyquist plots of copper in 0.5M sulphuric acid at different temperatures |
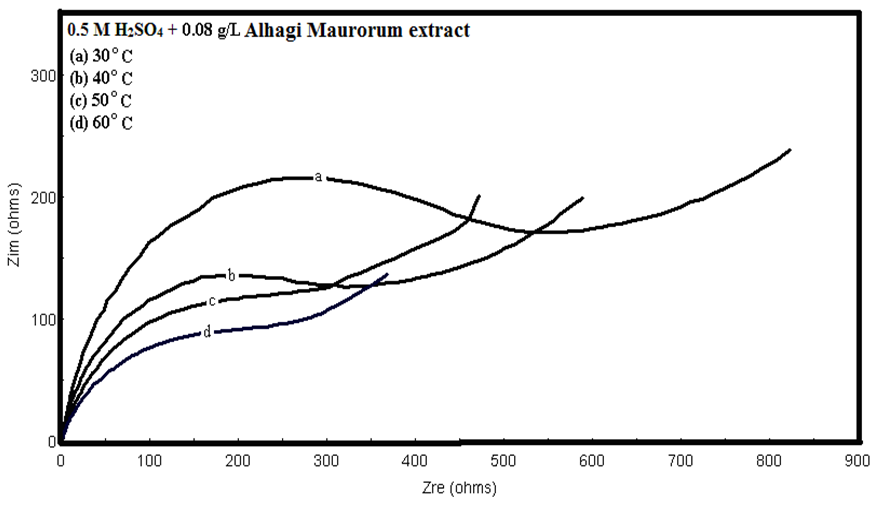 | Figure 11. Nyquist plots of copper in 0.5M sulphuric acid, in presence of 0.08 g/L of Alhagi Maurorum extract at different temperatures |
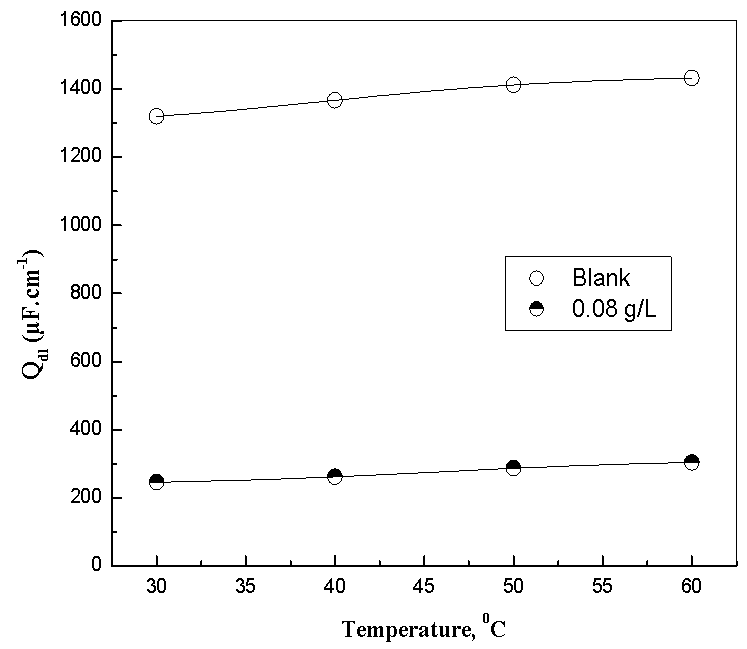 | Figure 12. Variation of the Cdl values in absence and presence of 0.08 g/L of Alhagi Maurorum extract at different temperatures |
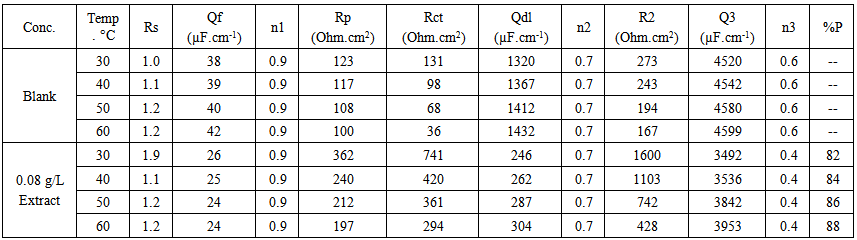 | Table 4. The electrochemical impedance parameters of copper in 0.5M sulphuric acid solution in absence and presence of 0.08 g/L Alhagi Maurorum extract at different temperatures |
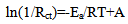 | (15) |
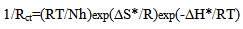 | (16) |
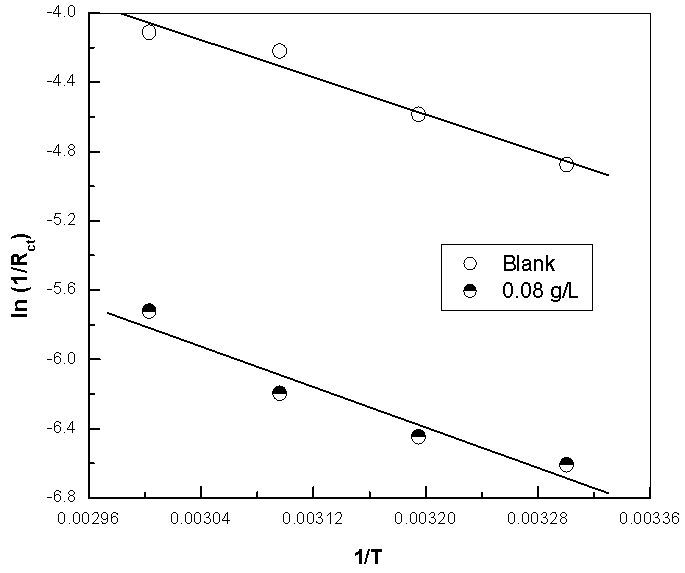 | Figure 13a. Linear Square fit for copper of ln 1/Rct vs. (1/T) |
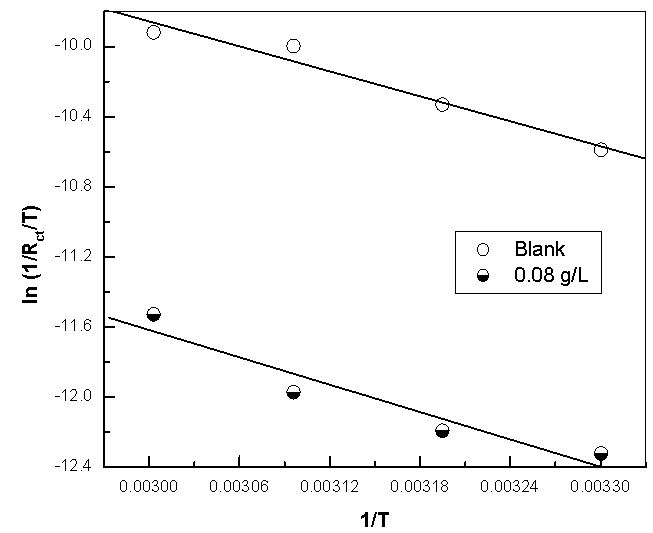 | Figure 13b. Linear Square fit for copper of ln((1/Rct)/T) vs. (1/T) |
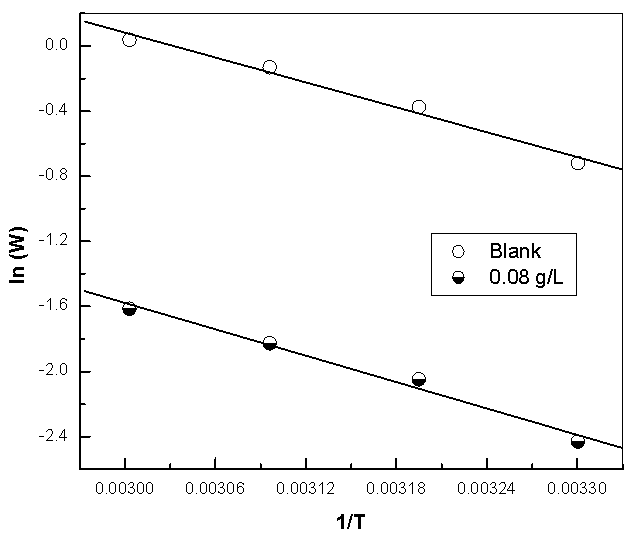 | Figure 14a. Linear Square fit for copper of ln W vs. (1/T) |
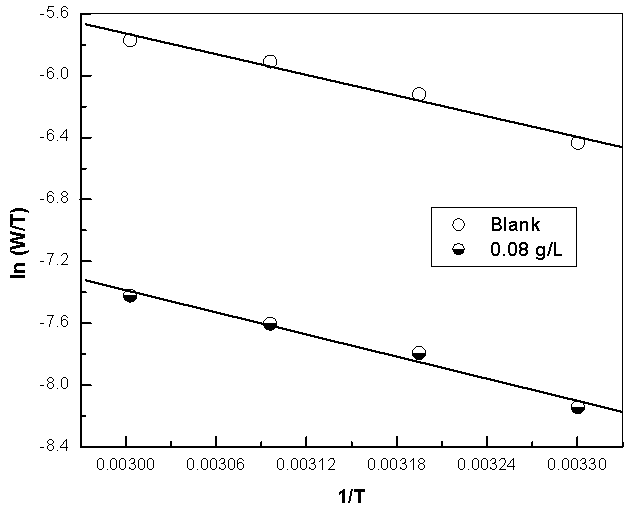 | Figure 14b. Linear Square fit for copper of ln((W)/T) vs. (1/T) |
3.6. Stability of the Extract
- The stock solution of the extract has been stored in refrigerator at 5°C. After certain times of storage, solution of 0.5M H2SO4 containing 0.08 g/L extract was prepared and Nyquist plots of copper in these solutions have been recorded (figure 15). As seen, the size of the capacitive semicircle slightly decreases with increasing the storage time. These plots were analyzed by the equivalent circuit that is previously used, Figure 4.Figure 15 shows the dependence of the percentage inhibition of Alhagi Maurorum extract (0.08 g/L) on the corrosion of copper in 0.5M H2SO4 on the storage time of the extract. This curve represents slightly decrease in the percentage inhibition with the storage time up to 40 days indicative of remarkable stability of the extract during the storage period.
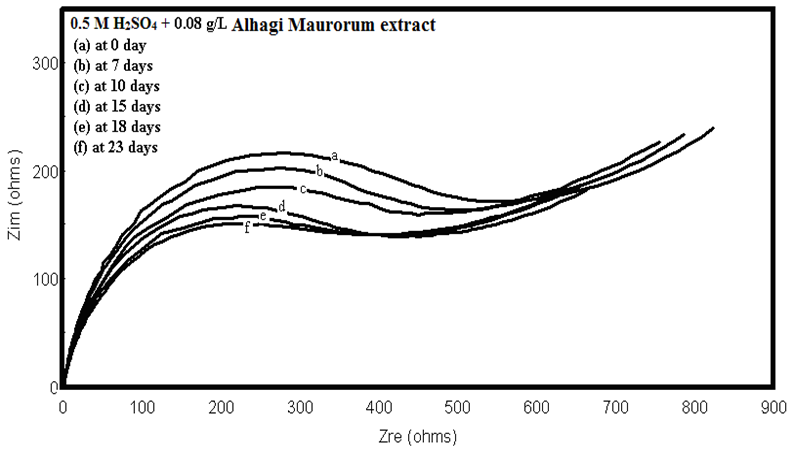 | Figure 15. Nyquist plots of copper in 0.5M sulphuric acid, in absence and presence of different concentrations of Alhagi Maurorum extract at different storage time |
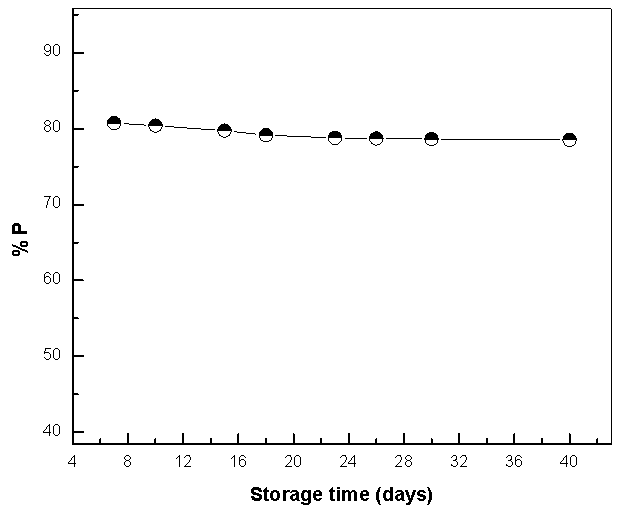 | Figure 16. Relation between the percentage inhibition efficiency the storage time of Alhagi Maurorum extract for copper in 0.5M sulphuric acid solution |
4. Conclusions
- 1. Potentiodynamic polarization results indicated that Alhagi Maurorum extract act as efficient cathodic type inhibitor for the corrosion of copper in 0.5M sulphuric acid and the efficiency of inhibition was found to increase with increasing the concentration of the extract. 2. EIS results showed that the dissolution process of copper occurs under diffusion control.3. Weight loss results showed that the inhibition efficiency of Alhagi Maurorum extract for the corrosion of copper in 0.5M sulphuric acid decreases slightly with increasing the immersion time in presence of low concentrations of the extract, but in presence of high concentrations of the extract the inhibition efficiency is nearly constant and independent of immersion time. 4. Langmuir, Flory-Hyggins isotherm, and Kinetic-Thermodynamic model were found to be applicable to fit the data of adsorption of Alhagi Maurorum extract at the copper surface. The data showed that the adsorbed species of Alhagi Maurorum extract are displacing only one water molecule.5. Impedance measurements at different temperatures showed that the inhibition efficiency of the Alhagi Maurorum extract for the corrosion of copper in 0.5M sulphuric acid increases slightly to 82 - 86% at temperatures 30 - 60°C respectively indicative that Alhagi Maurorum extract is a good inhibitor.6. The activation parameters of the corrosion reaction of copper in acidic medium are measured using the weight loss method and impedance technique, a fairly good agreement between the values of Ea , ΔH* and ΔS* is obtained.
ACKNOWLEDGEMENTS
- I wish to express my deep gratitude to Prof. Dr. Beshir Ahmed Abd-EL- Nabey, Professor of Physical Chemistry, Chemistry Department, Faculty of Science, Alexandria University, for his guidance and constructive criticism during the various stages of this work and finalizing the paper in its present form.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML