-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Physical Chemistry
p-ISSN: 2167-7042 e-ISSN: 2167-7069
2015; 5(2): 34-38
doi:10.5923/j.pc.20150502.02
First-Principles Study on Anatase Co/TiO2: Effect of Co Concentration
Fatemeh Mostaghni1, Yasaman Abed2
1Chemistry Department, Payam Noor University, Iran
2Physic Department, Payam Noor University, Iran
Correspondence to: Fatemeh Mostaghni, Chemistry Department, Payam Noor University, Iran.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This study was performed to comprehend the systematic analysis of the cobalt impurity states in different concentrations as well as their influence on the TiO2 band structure and density of states. For thisporpuse, first principle plane wave pseudo-potential technique based on density functional theory with CASTEP module were used. The calculations showed that band gap of Co-doped TiO2 decreases with an optimal doping concentration of 20% as a result of the overlap among the Co 3d, Ti 3d, and O 2p states, which enhances photocatalytic activity in the visible light region.
Keywords: Molecular simulation, Castep, Band stracture, Density of states
Cite this paper: Fatemeh Mostaghni, Yasaman Abed, First-Principles Study on Anatase Co/TiO2: Effect of Co Concentration, Physical Chemistry, Vol. 5 No. 2, 2015, pp. 34-38. doi: 10.5923/j.pc.20150502.02.
Article Outline
1. Introduction
- Anatase TiO2 became the most widely investigated semiconductor in many fields such as solar cells, air and water pollution controlling. The anatase phase TiO2 has been employed the photocatalytic activity only under the ultraviolet (UV) light illumination due to its large indirect band gap ca 3.2 eV. Considerable research has gone into modifying the band gap of TiO2. One of the most effective methods involves Dopping of transition metal ions into TiO2 [1-9].Since then, different metals have been doped into TiO2 nanomaterials. Commonly assumed knowledge says that the noble metal ion acts as a sink for photoinduced charge carriers, and this promotes interfacial charge-transfer processes. Li et al. developed La3+-doped TiO2 via sol–gel and found that lanthanum doping inhibits phase transformation of TiO2, enhances its thermal stability, reduces its crystallite size, and increases Ti3+ surface content [10]. Bessekhouad et al. investigated Li, Na and K doped TiO2 nanoparticles and found that the crystalline levels were largely dependent on both the nature and the concentration of the alkaline. [11].Doping an element with TiO2 is considered as the feasible and effective way of restructuring the bandgap of TiO2. The doping of semiconductors changes the probabilities for charge carriers to participate in conduction. This probability were quantified by the density of states within the semiconductor as a function of electron energy. This density of states combined with the probability distribution of a state at a particular energy being occupied will determine the overall density of charge carriers as a function of energy. In order to quantify the possible energy states available in a quantum mechanical system we need solutions to Shrodinger's equation. However, the theoretical analysis by computer simulation could clarify the ion doping effects on crystal and electronic structure. The use of quantum mechanical calculations to accurately predict the structure and properties of crystals is going to have significant implications for the studies of semiconductors.CASTEP is a first principles electronic structure code for predicting properties of materials. It employs the density functional theory plane-wave pseudopotential method, which allows to perform first-principles quantum mechanics calculations that explore the properties of crystals and surfaces in materials such as semiconductors, ceramics, metals, minerals, and zeolites [12-14].Ashahi et al have performed first-principles calculations using the full-potential linearized augmented plane-wave method to investigate detailed electronic and optical properties of TiO2 in the anatase structure [15].Segall et al have studied the band structures and charge densities of nitrogen, carbon and boron doped TiO2 by first-principles simulation with the CASTEP code [16]. Andriyevsky et al have studied Electronic band structure, density of states and complex dielectric function of KDP crystal at Fdd2 and space groups of symmetry corresponding to the ferroelectric and paraelectric phases, have been calculated within the density functional theory using the VASP code. The experimental data and theoretically calculated dielectric functions have demonstrated a good agreement [17].To obtain insight into this issue, we have performed a careful and systematic analysis of the cobalt impurity states in different concentrations as well as their influence on the TiO2 band structure and density of states.
2. Computational Details
- All presented calculations were performed using a first principle plane wave pseudo-potential technique based on density functional theory with CASTEP module [13] of Materials Studio. The density functional is treated by GGA with the exchange correlation potential parameterization. Figure 1 shows the supercell structures of crystalline Co-doped TiO2. The pure supercell was monodoped with cobalt in place of titanium.
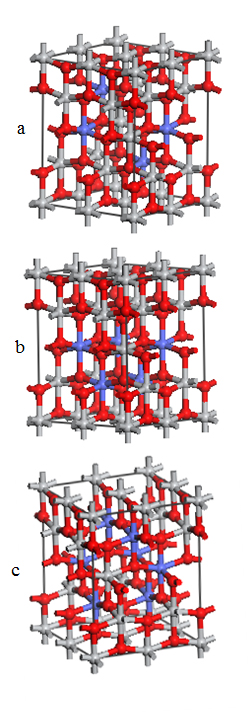 | Figure 1. 2 × 2 × 1 supercell models with various concentrations of Co: (a) 15%, (b) 20%, (c) 25% |
3. Result and Discussion
- Anatase TiO2 has a tetragonal structure with two lattice parameters a = b = 3.785 Å and c = 9.514 Å[14] and belongs to the space group 141/amd.In this study, first anatase unit cell was optimized and its lattice parameter are a = b = 3.791 Å and c= 9.63Å. The energy band structure and density of states (DOS) for pure anataseTiO2 is obtained within the GGA approximations that are shown in Figures 2a and 3a respectively.Then, Co dopant is substituted into anatase structure in Ti lattice site in various concentrations( 15, 20 and 25%), once again the structure was optimized. Total energy of doped structures shows that they are energetically favourable. The valence configurations of the atoms were 3s23p63d24s2 for Ti, 2s22p4 for O, and 3d74s2 for Co.Figure 2a shows that the top of valence bands (VBs) appears to be relatively flat and the bottom of conduction bands (CBs) have small dispersion. The Fermi level, indicated by a dotted line, is also valence band maximum and was set to zero. The band structure of pure anatase TiO2 shows semiconducting behavior because in this case direct energy band gap is 2.29 eV. It is less than the experimental value Eg = 3.23 eV. this underestimation is an intrinsic feature of the generalized-gradient approximation (GGA).In figure 3a, we can also clearly see the conduction bands, are mostly formed by Ti-4d state, with a contribution of the O-3 p states, while the valance bands are mainly consist of the hybridizations of Ti-3d states and O-2p states. Using the first-principle calculations the electronic structures and band parameters for Co/TiO2 in varius concentration (15, 20 and 25%) are obtained within the same approximations. The energy band structure and density of states (DOS) of Co/TiO2 in concentration 15, 20 and 25% are shown in Figures. 2b-d and 3b-d respectively.Figure 2 and 3 shows the substitutional Co dopant created impurity states in electronic band structure of anatase. The Co introduced two lone pair states just above to the valence band maximum thus reduced the band gap of TiO2 with an optimal doping concentration of 20%.
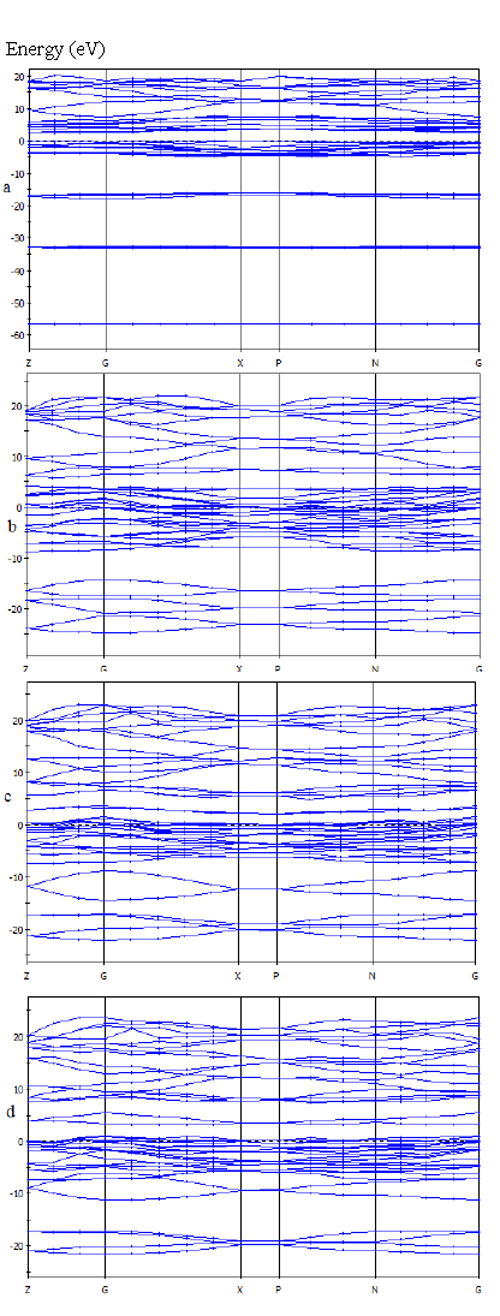 | Figure 2. Band stracture of undoped TiO2 (a) and TiO2 doped with Co 15% (b), 20% (c), 25% (d). The horizontal dotted line is the Fermi energy level |
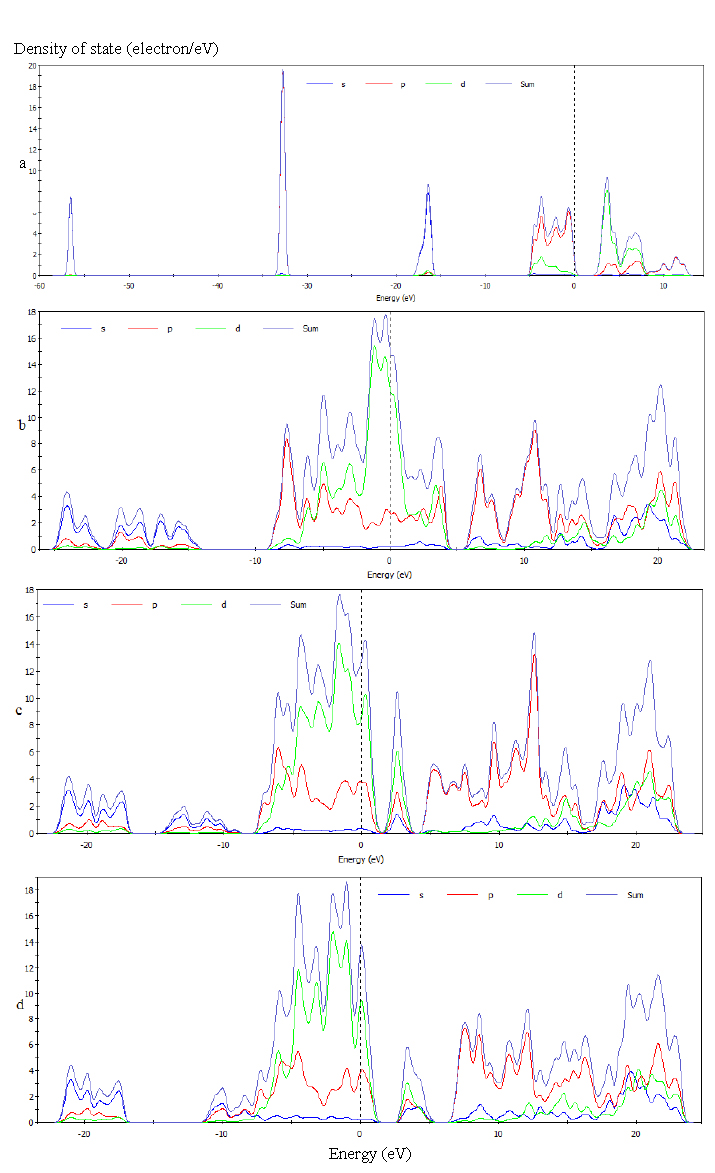 | Figure 3. Density of states (DOS) of undoped TiO2 (a) and TiO2 doped with Co 15 % (b), 20 % (c), 25 % (d). The vertical dotted line is the Fermi energy level |
|
4. Conclusions
- The DOS and band structure of cobalt doped TiO2 indicated that the cobalt dopant induced the band gap narrowing. Thus the performance of TiO2 can be enhanced by shifting the absorption spectrum into the visible region. It is apparent that the band gap shifts towards lower values with the increase in Co concentrations. The decreasing trend of band gap with the increasing dopant level indicates that more number of free electrons is available for charge conduction.In addition, the variation of the band gap showed the band gap is greatly sensitive to the dopant concentration. It is observed that, higher concentrations of cobalt facilitate conductivity of TiO2. It is important to note that, at moderate level of doping (20%) exhibits the lowest band gap. Further increase in dopant concentration until 25% results in larger band gap. Therefore the best concentration of cobalt dopant was 20% based on the first-principles calculations.
ACKNOWLEDGEMENTS
- We would like to thank Fars Payame Noor University research council for the financial support (Grant # 1393/7/48910).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML