-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Physical Chemistry
p-ISSN: 2167-7042 e-ISSN: 2167-7069
2015; 5(1): 1-5
doi:10.5923/j.pc.20150501.01
Density and Partial Molar Volume of Cetyltrimethylammonium Bromide in the Presence and Absence of KCl and NaCl in Aqueous Media at Room Temperature
Ajaya Bhattarai , Sujeet Kumar Chatterjee , Kabita Jha
Department of Chemistry, Mahendra Morang Adarsh Multiple Campus, Tribhuvan University, Biratnagar, Nepal
Correspondence to: Ajaya Bhattarai , Department of Chemistry, Mahendra Morang Adarsh Multiple Campus, Tribhuvan University, Biratnagar, Nepal.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
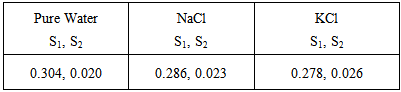
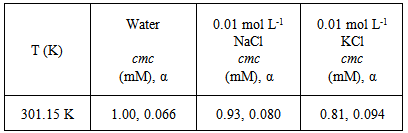
The density of cetyltrimethylammonium bromide in pure water and in the presence of KCl and NaCl at room temperature are reported. The concentrations of cetyltrimethylammonium bromide are varied from (0.013 to 0.0002) mol L-1. Both the salts have a concentration of 0.01 M. The density of cetyltrimethylammonium bromide in presence of KCl is higher than NaCl. On the basis of the obtained results of density measurements, the critical micelle concentration (cmc), degree of dissociation (α) and partial molar volume of cetyltrimethylammonium bromide in pure water and in the presence of KCl and NaCl are determined. The obtained cmc values are also analyzed with those accounted on the basis of the surface tension data from the previous paper [1]. It is observed that the partial molar volume of surfactant increases with increase in concentration. The lesser value of partial molar volumes of cetyltrimethylammonium bromide is noticed in the presence of KCl than NaCl.
Keywords: Cetyltrimethylammonium bromide, KCl, NaCl, Density, Partial molar volume, Degree of dissociation
Cite this paper: Ajaya Bhattarai , Sujeet Kumar Chatterjee , Kabita Jha , Density and Partial Molar Volume of Cetyltrimethylammonium Bromide in the Presence and Absence of KCl and NaCl in Aqueous Media at Room Temperature, Physical Chemistry, Vol. 5 No. 1, 2015, pp. 1-5. doi: 10.5923/j.pc.20150501.01.
Article Outline
1. Introduction
- When the surfactants are mixed with water they distort the structure of water and thereby increase the free energy of the system. Therefore, they concentrate at the surface and are so orientated that their hydrophobic groups “turn away” from the solvent, and the free energy of the solution is minimized. On the other hand, the distortion of the solvent structure can also be decreased by aggregation of the surface-active molecules into micelles with their hydrophobic groups directed towards the interior of the micelle, and their hydrophilic groups directed towards the solvent. Micellization is therefore an important mechanism in relation to adsorption at interfaces for removing hydrophobic groups from their contact with water, reducing thereby the free energy of the system [2-4]. Since the properties of surfactant solutions change markedly when micelle formation occurs, many investigations have been focused on determining the values of the cmc in various systems and many studies have been carried out to elucidate the factors that determine the cmc value at which micelle formation becomes significant, especially in aqueous media. The most important factor known to affect the cmc in aqueous solution is the structure of a surfactant [5].Volumetric, viscometric, and other thermodynamic data provide valuable information regarding solute-solvent, solute-solute, and solvent-solvent interactions [6, 7]. Among various physical parameters, density and partial molar volume have been recognized are the quantities that are sensitive to structural changes occurring in solutions [8]. The partial molar volume, , is defined by Wandrey et al. [9], as the following equation;
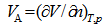 | (1) |
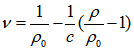 | (2) |
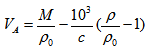 | (3) |
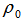 is the density of the solvent,
is the density of the solvent, 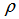 is the density of the solution and c is the equivalent concentration in mol L-1.In order to calculate partial molar volumes, the solution densities are measured for cetyltrimethylammonium bromide in pure water and in the presence of NaCl and KCl at the room temperature.In this work, the results are reported for density measurements on cetyltrimethylammonium bromide, a cationic surfactant, in the presence and absence of salts (NaCl and KCl) at room temperature. The aim of the present work is to analyze the influence of concentration and salts on cetyltrimethylammonium bromide in aqueous media for density and also see the influence of concentration and salts for partial molar volumes for cetyltrimethylammonium bromide.Therefore, the purpose of our article is to compare the cmc of cetyltrimethylammonium bromide in the presence of salts on the basis of surface tension measurements [1] with density methods and the calculation of degree of dissociation for cetyltrimethylammonium bromide in the presence of salts.
is the density of the solution and c is the equivalent concentration in mol L-1.In order to calculate partial molar volumes, the solution densities are measured for cetyltrimethylammonium bromide in pure water and in the presence of NaCl and KCl at the room temperature.In this work, the results are reported for density measurements on cetyltrimethylammonium bromide, a cationic surfactant, in the presence and absence of salts (NaCl and KCl) at room temperature. The aim of the present work is to analyze the influence of concentration and salts on cetyltrimethylammonium bromide in aqueous media for density and also see the influence of concentration and salts for partial molar volumes for cetyltrimethylammonium bromide.Therefore, the purpose of our article is to compare the cmc of cetyltrimethylammonium bromide in the presence of salts on the basis of surface tension measurements [1] with density methods and the calculation of degree of dissociation for cetyltrimethylammonium bromide in the presence of salts.2. Experimental
- Triply distilled water with a specific conductance less than 10-6 S.cm-1 at 308.15 K was used for the preparation of the solution. Cetyltrimethylammonium bromide was purchased from Loba Chemical Private Limited, India and it was recrystallised several times until no minimum in the surface tension-concentration plot was observed and its critical micellar concentration (cmc) agreed with the literature value [11]. Both the salts (NaCl and KCl) was purchased from Ranbaxy India Ltd and making the solution of same concentration of 0.01 M.To measure density the pycnometeric method was used. The stock solutions were freshly prepared for each concentration series to avoid problems of aging and microorganism contamination, which was found to occur with diluted surfactant solutions [12]. The densities of solutions were determined by the use of Ostwald-Sprengel type pycnometer of about 10 cm3 capacity. The sample solution was transfused into the pycnometer by using a medical syringe. The mass of the pycnometer was measured with electronic balance and the density was calculated. Density measurements are believed to be precise within ± 0.00005, which is satisfactory for our purpose. In order to avoid moisture pickup, all solutions were prepared in a dehumidified room with utmost care. In all cases, the experiments were performed at least in three replicates.The partial molar volume was calculated from the equation (3) by using molecular weight of cetyltrimethylammonium bromide(M) is 364.43, the density of the solvent (pure water) 0.996237 at 301.15K which was obtained from the literature [13].
3. Results and Discussion
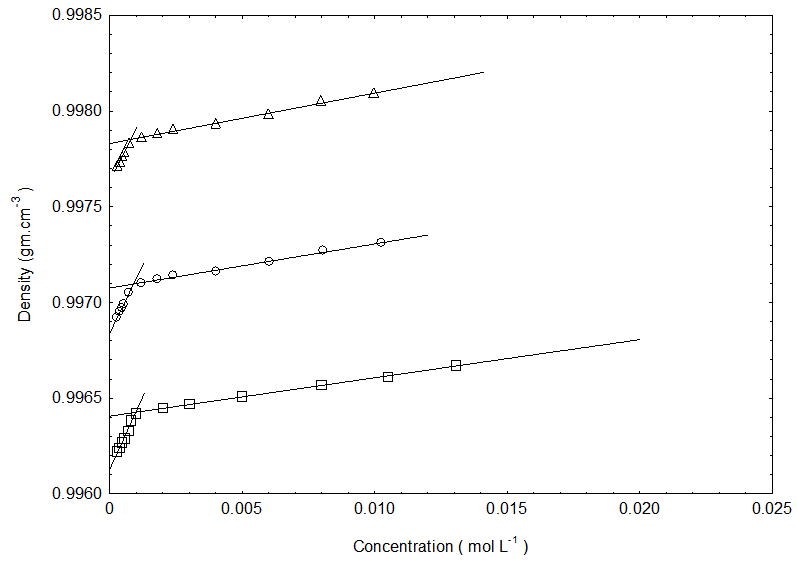
- The densities for the cetyltrimethylammonium bromide in the absence and presence of NaCl and KCl in pure water at room temperature is depicted in Figure 1. Figure 1 shows the variation of densities of the investigated solutions as a function of the surfactant concentrations. From this figure it is evident that the densities exhibit almost increase with increasing concentration within the concentration range investigated here. Our density data of cetyltrimethylammonium bromide in the presence of salts(NaCl and KCl) in pure water is found to be higher than the density of cetyltrimethylammonium bromide in pure water. The increase in density with the addition of KCl is in agreement with literature [14]. Moreover, the density of cetyltrimethylammonium bromide in pure water in the presence of KCl is higher than NaCl.
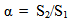 | (3) |
|
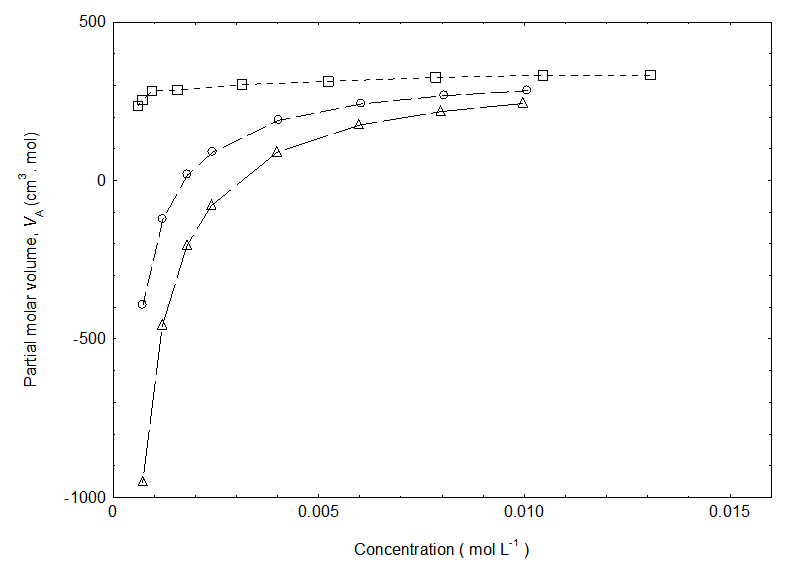 | Figure 2. Partial molar volume of cetyltrimethylammonium bromide in pure water (square), in the presence of NaCl(circle) and KCl(triangle) at 301.15K |
- As we know that the higher degree of dissociation results in an increase of the specific conductivity [20]. In the presence of KCl, the degree of dissociation of CTAB is high because KCl has a higher specific conductivity than NaCl [21].
4. Conclusions
- The results showed that the density of cetyltrimethylammonium bromide increases with increase of concentrations. The density of cetyltrimethylammonium bromide in the presence of KCl found higher than NaCl. The partial molar volume of cetyltrimethylammonium bromide increases with increasing surfactant concentration. Also, the partial molar volumes decrease in the presence of salts. The lesser value of partial molar volumes of cetyltrimethylammonium bromide is noticed in the presence of KCl than NaCl. It is found that the cmc of cetyltrimethylammonium bromide deceases with the addition of salts. The cmc of cetyltrimethylammonium bromide decreases more in the presence of KCl in comparison with the presence of NaCl. In the presence of KCl, the degree of dissociation of CTAB is high than NaCl.
ACKNOWLEDGEMENTS
- One of the authors (Kabita Jha) is thankful to the University Grants Commission (UGC), Nepal, for providing grants for M. Sc. Dissertation work and the authors are grateful to Associate Professor G.S. Shrivastav, Head of the department of Chemistry, Mahendra Morang Adarsh Multiple Campus, Tribhuvan University, Biratnagar for providing the research facilities to conduct this research.
References
| [1] | K. Jha, A. Bhattarai, S.K. Chatterjee, Surface tension studies on the micellization of cetyltrimethylammonium bromide in presence and absence of KCl and NaCl in aqueous media at room temperature, BIBECHANA 10(2014) 52-57. |
| [2] | J. M. Rosen, Surfactants and Interfacial Phenomena, Wiley–Interscience, New York, 1989, Chap. 6. |
| [3] | K. Holmberg, B. Jonnson, B. Kronberg, B. Lindman, Surfactants and Polymers in Aqueous Solution, second ed., Wiley, England, 2003. |
| [4] | J. Eastoe, J. S. Dalton, Dynamic surface tension and adsorption mechanisms of surfactants at the air–water interface, Adv. Colloid Interface Sci. 85 (2000) 103-144. |
| [5] | J. Harkot, B. Janczuk, Surface and volume properties of dodecylethyldimethylammonium bromide and benzyldimethyldodecylammonium bromide, J. Colloid & Interface Sci. 330(2009)467-473. |
| [6] | M. R. J. Dack, K. J. Bird, A. J. Parker, Solvation of ions. XXV. Partial molal volumes of single ions in protic and dipolar aprotic solvents, Aust. J. Chem. 28(1975)955-963. |
| [7] | J.M. Mc Dowali, C.A. Vincent, Viscosity behaviour of some simple electrolytes in formamide solution, J. Chem. Soc. Faraday Trans. (1974)1862-1868. |
| [8] | M. F. Hossain, T. K. Biswas, M. N. Islam, M. E. Huque, Volumetric and viscometric studies on dodecyltrimethylammonium bromide in aqueous amino acid solutions in premicellar region, Monatsh Chem. 141 (2010)1297-1308. |
| [9] | C. Wandrey, A. Bartkowiak, D. Hunkeler, Partial molar and specific volumes of polyelectrolytes: comparision of experimental and predicted values in salt-free solutions, Langmuir 15(1999) 4062-4068. |
| [10] | R. De Lisi, S. Milioto, R. E. Verrall, Partial molar volumes and compressibilities of aikyltrimethylammonium bromides, J. Solution Chem. 19(1990) 665- 692. |
| [11] | T. Chakraborty, I. Chakraborty, S. Ghosh, Sodium carboxymethylcellulose-CTAB interaction: A detailed thermodynamic study of polymer-surfactant interaction with opposite charges. Langmuir 22(2006) 9905-9913. |
| [12] | A. Domard, M. Rinaudo, Preparation and Characterization of fully deacetylated Chitosan, Int. J. Biol. Macromol, 1983, 5, 49-52. |
| [13] | R. C. Weast, Handbook of Chemistry and Physics, CRC press, 64th Ed., 1984. |
| [14] | N. Takenaka, T. Takemura, Partial Molar Volumes of Uni-univalent electrolytes in Methanol + water. 1. Lithium Chloride, Sodium Chloride, and Potassium Chloride, J. Chem. Eng. Data, 39(1994)207-213. |
| [15] | M. Usman, A. Khan, M. Siddiq, Thermodyanmic and Solution properties of Amphiphilic Anti-Allergic Drug Cetirizine HCl, J. Chem. Soc. Pak.,31(2009)221-227. |
| [16] | R. Zana, Ionization of cationic micelles: Effect of the detergent structure, J. Colloid Interface Sci. 78 (1980) 330-337. |
| [17] | K. Manna, A. Panda, Physicochemical Studies on the Interfacial and Micellization behaviour of CTAB in Aqueous Polyethylene Glycol Media, J. of Surfact. Detergent, 14 (2011)563-576. |
| [18] | C. Oelschlaeger, N. Willenbacher, Mixed wormlike micelles of cationic surfactants: Effect of the cosurfactant chain length on the bending elasticity and rheological properties, Colloids and Surfaces A: Physicochem. Eng. Aspects 406 (2012) 31– 37. |
| [19] | K.U. Din, R.M. Abdul, N. Z. Andleeb, Effect of Inorganic Salts and Ureas on the Micellization Behavior of Antidepressant Drug Imipramine Hydrochloride at Various Concentrations and Temperatures, Acta Phys. Chim. Sin. 28(2012)885-891. |
| [20] | D.V.S. Murty, Transducers and Instrumentation, Second Edition, PHI Learning Pvt. Ltd., Technology & Engineering, page 318, 2010. |
| [21] | A. Bhattarai, S.K. Chatterjee, K. Jha, Thermodynamics Studies Associated with Micellization of Cetyltrimethylammonium Bromide in the Presence and Absence of KCl and NaCl in Aqueous Media, International Journal of Latesh Research in Science and Technology Vol. 3 (2014) 211-213. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML